Obesity in pregnant women induces fetal cardiac dysfunction and raises the risk of offspring cardiovascular disease, although its influence on myocardial metabolism is uncertain. Maternal obesity has significant long-term consequences for offspring health, including obesity and cardiovascular issues. Obesity is caused by a number of variables, including the interaction of heredity and environment.
According to a new mouse study, maternal obesity harms the fetus’s heart health and function. The study, published in The Journal of Physiology, discovered that maternal obesity produces molecular changes in the heart of the fetus and modifies the expression of genes associated to nutrition metabolism, dramatically increasing the offspring’s risk of cardiac issues later in life.
This is the first study to show that the nutrients received during fetal life ‘programme’ the heart. Gene expression changes affect how the heart typically metabolizes carbs and lipids. They change the heart’s nutritional choice away from sugar and toward fat. As a result, the hearts of fat female mouse fetuses were larger, weighed more, had thicker walls, and had symptoms of inflammation. This reduces the efficiency with which the heart contracts and pumps blood throughout the body.
The researchers from the University of Colorado in the United States employed a mouse model that mimics human maternal physiology and placental nutrition transfer in obese women. Female mice (n=31) were fed a diet with a high fat content together with a sugary drink, which is equivalent to a human regularly consuming a burger, chips and a fizzy drink (1500kcal). The female mice ate this diet until they developed obesity, putting on about 25% of their original body weight. 50 female mice were fed a control diet.
Our findings point to a relationship between maternal obesity and cardiometabolic disease in the next generation. This is significant since obesity is quickly growing in the human population, affecting nearly one-third of women of reproductive age.
Dr Owen Vaughan
Mouse pups (n=187) were evaluated in utero, after birth, and at 3, 6, 9, and 24 months utilizing imaging techniques such as echocardiography and positron emission tomography (PET) scans. Researchers examined the offspring’s DNA, proteins, and mitochondria.
The effects of sex on offspring heart metabolism were significant. The expression of 841 genes was altered in female foetuses’ hearts and 764 genes were affected in male foetuses’ hearts, although less than 10% of genes were altered in both sexes. Although both male and female offspring of obese mothers had decreased cardiac function, there were disparities in the advancement of the sexes; males were damaged from the start, whereas females’ cardiac function deteriorated with age.
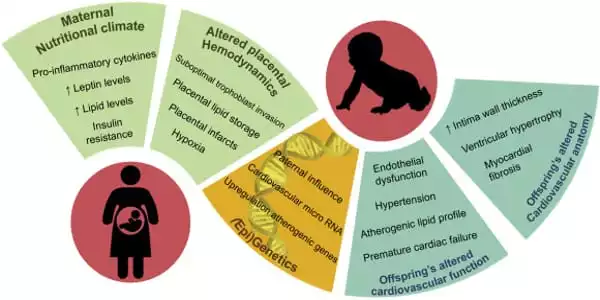
The sex-difference in the lasting impairments of cardiovascular health and function could be due to oestrogen. Higher levels in young females may protect cardiovascular health, the protection diminishes as oestrogen levels deplete as the females age. The molecular cause for the sex difference is not yet understood.
Lead author, Dr Owen Vaughan, University of Colorado, US said:
“Our findings point to a relationship between maternal obesity and cardiometabolic disease in the next generation. This is significant since obesity is quickly growing in the human population, affecting nearly one-third of women of reproductive age. This discovery paves the way for medicines that could be utilized in early life to prevent later-life cardiometabolic disorders, which are costly for health services and have a negative impact on many people’s quality of life. For example, we could provide more targeted nutrition advice to mothers or children based on their BMI or gender, or we could create novel medications that target metabolism in the fetus’ heart.”
Untreated obesity in the prenatal period lays the groundwork for a slew of symptoms and unpleasant delivery experiences, such as gestational hypertensive disorders, gestational diabetes, macrosomia, and labor difficulties. However, findings from human and animal studies suggest that nutritional treatments and physical activity can mitigate many of the negative consequences of obesity on offspring metabolic health.
Mice have shorter pregnancies, more offspring, and a different diet than humans, thus further research in human volunteers is needed to extend the findings to women’s health. Loss-of-function studies are also required to verify this pathway between maternal fat and offspring cardiac function and to determine the particular molecules implicated.