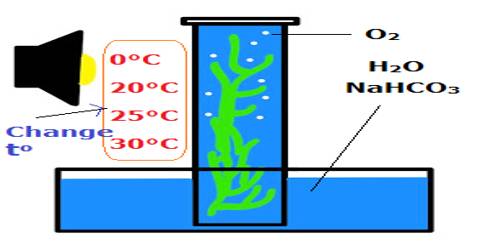
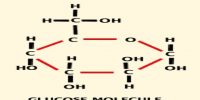
Task: To observe the affect of temperature on solution.
Required accessories: One beaker, stirrer, tripod stand, wire-net, one balance, salt, water and spirit lamp.
Procedure: Use the balance to take 100 gm water in a clean beaker. Adding salt to in little amounts and continues to stir. Stop adding salt when the added salt is no more dissolving. Now, place the beaker on the wire net kept aver the tripod and apply heat at the bottom of the beaker by the spirit lamp and continue to stir the solution.
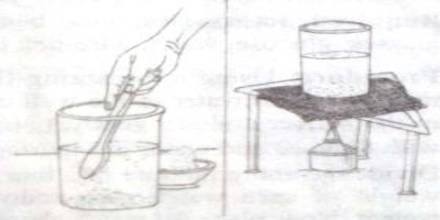
Analysis: For many solids dissolved in liquid water, the solubility increases with temperature. The increase in kinetic energy that comes with higher temperatures allows the solvent molecules to more effectively break apart the solute molecules that are held together by intermolecular attractions.
Do you observe any change in the solution alter applying heat? Yes, slowly the amount of un-dissolved salt begins to decrease. In this way, with the heating up of the solution for some time, the entire salts will he dissolved. Then it can be said that, the solubility of salt in water has been increased due to increase of temperature. For this reason the un-dissolved salt got dissolved after the solution was heated. But in case of some solutions (for example, cerium-sulphate and calcium hydro-oxide in water), solubility of the solute decreases with the increase of temperature.