Pancreatic cancer starts in the tissues of your pancreas, which is an organ in your abdomen located behind the lower part of your stomach. Your pancreas produces hormones that help you manage your blood sugar and secretes enzymes that aid digestion. Pancreatic cancer is rarely discovered in its early stages when it is most treatable. This is due to the fact that it frequently does not cause symptoms until it has spread to other organs.
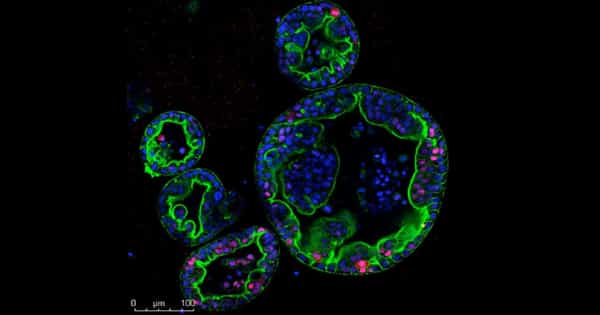
In the laboratory, an international team of scientists created a three-dimensional (3D) pancreatic cancer tumor model using a bioengineered matrix and patient-derived cells that could be used to develop and test targeted treatments.
Researchers from the University of Nottingham, Queen Mary University of London, Monash University, and Shanghai Jiao Tong University created a multicellular 3D microenvironment using patient-derived cells to recreate the way tumor cells grow in pancreatic cancer and respond to chemotherapy drugs in a new study published today in Nature Communications.
Pancreatic cancer is extremely difficult to treat, especially because there are no symptoms until cancer has spread. It can be resistant to treatment, and the survival rate is low when compared to other cancers, with only a 5-10% five-year survival rate.
An international team of scientists have created a three-dimensional (3D) pancreatic cancer tumour model in the laboratory, combining a bioengineered matrix and patient-derived cells that could be used to develop and test targeted treatments.
The study was led by Professors Alvaro Mata from the University of Nottingham (UK), Daniela Loessner from Monash University (Australia), and Christopher Heeschen from Shanghai Jiao Tong University (China). Dr. David Osuna de la Peña, a lead researcher on the project, said: “There are two major obstacles to treating pancreatic cancer: a dense protein matrix and the presence of highly resistant cancer stem cells (CSCs) involved in relapse and metastasis. In our research, we created a matrix in which CSCs can interact with other cell types and behave more as they do in the body, allowing us to test different treatments in a more realistic setting.”
Improved 3D cancer models are required to study tumor growth and progression in patients as well as test responses to new treatments. Currently, 90% of successful cancer treatments tested pre-clinically fail in the early stages of clinical trials, and less than 5% of oncology drugs are successful in clinical trials.
To predict treatment responses, pre-clinical tests primarily rely on a combination of two-dimensional (2D) lab-grown cell cultures and animal models. Conventional 2D cell cultures, on the other hand, fail to mimic key features of tumor tissues, and interspecies differences can render many successful treatments in animal hosts ineffective in humans.
As a result, novel experimental 3D cancer models are required to better recreate the human tumor microenvironment and account for patient-specific differences. The process by which biological systems controllably assemble multiple molecules and cells into functional tissues is known as self-assembly. Using this process, the researchers developed a new hydrogel biomaterial composed of multiple, yet distinct, proteins found in pancreatic cancer. This mechanism of the formation allows for the incorporation of key cell types in order to create biological environments that can mimic the characteristics of a patient’s tumor.
“Using models of human cancer is becoming more common in developing treatments for the disease, but a major barrier to getting them into clinical applications is the turnaround time,” Professor Mata adds. By assembling and organizing key matrix components with patient-derived cells, we created a comprehensive and tuneable ex vivo model of pancreatic ductal adenocarcinoma (PDAC). The models have patient-specific transcriptional profiles, CSC functionality, and high tumorigenicity, making them more relevant than Organoid and Sphere cultures. Most importantly, our self-assembled cultures reproduced drug responses better than the other models.
We believe that this model brings us closer to our goal of being able to take patient tumor cells in the hospital, incorporate them into our model, find the best cocktail of treatments for specific cancer, and deliver it back to the patient in a timely manner. Although precision medicine for treating this disease is still a long way off, this research is a step in the right direction.”