The aromatic amines are an industrially significant class of organic compounds being extensively used in the production of compounds and intermediates for the dyestuff, rubber-processing, and drug-manufacturing industries.
Physical properties of Aromatic Amine:
Aromatic amines have their lone pair electrons conjugated (“shared”) into the benzene ring, so their tendency to engage in hydrogen bonding is somewhat diminished. The boiling points of these molecules are therefore usually somewhat higher than other, smaller amines due to their typically larger size.
(i) Physical state: Aromatic amines are colorless liquid or solid compounds having an unpleasant odor. They become brown colored by oxidation with light or air. They are poisonous or toxic. Readily formed aniline is an oily liquid.
(ii) Melting point & Boiling Point: M.P & B.P increase with the rise of molecular mass. The melting point & boiling point of aromatic amines are higher than that of aliphatic ones.
(iii) Solubility: They are slightly soluble in water but completely soluble in organic solvents. Aniline is more soluble in water than the n-hexyl amine.
(iv) Hydrogen bonding: Hydrogen bonding significantly influences the properties of primary and secondary amines. Thus the boiling point of amines is higher than those of the corresponding phosphines but generally lower than those of the corresponding alcohols. For example, methylamine and ethylamine are gases under standard conditions, whereas the corresponding methyl alcohol and ethyl alcohols are liquids. Gaseous amines possess a characteristic ammonia smell, liquid amines have a distinctive “fishy” smell.
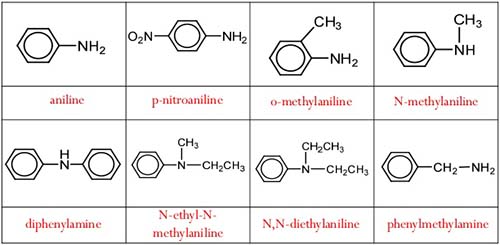
Some of the common naming of aromatic amines
Also reflecting their ability to form hydrogen bonds, most aliphatic amines display some solubility in water. Solubility decreases with the increase in the number of carbon atoms. Aliphatic amines display significant solubility in organic solvents, especially polar organic solvents.
Physical Properties of Amines
- The lower aliphatic amines are gaseous in nature. They have a fishy smell.
- Aniline and other arylamines are generally colorless.
- Lower aliphatic amines can form hydrogen bonds with water molecules.
- An increase in the size of the hydrophobic alkyl part increases the molar mass of amines.
- Higher amines are insoluble in water.
- Alcohols have higher polarity as compared to amines and hence, they form stronger intermolecular hydrogen bonds.
- The order of the boiling point of amines is as follows: Primary > Secondary > Tertiary.












