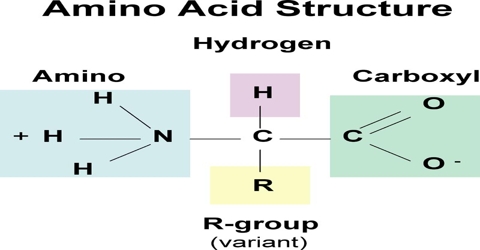
Proteins are complex nitrogenous organic compounds which are linked together by the peptide bond. It is a condensation polymer, polyamide. Proteins are the group of complex organic macromolecules that contain carbon, hydrogen, oxygen, nitrogen, and usually sulfur and are composed of one or more chains of amino acids. are made up of hundreds or thousands of smaller units called amino acids, which are attached to one another in long chains.
The composition of Protein: 05 primary elements:
(i) Carbon 53 %
(ii) Hydrogen 7 %
(iii) Oxygen 23 %
(iv) Nitrogen 16 %
(v) Sulphur 1 %
Proteins are the polymer of amino acid.
In 1954, Scientist Sanger determined the structural formula of insulin → a polypeptide having 51 molecules of 17 different amino acids, arranged in A & B chain. A chain contains 21 amino acid molecules & B-Chain contains 30 ammo acid molecules, both chains are linked together by a disulfide bond.
Linus Pauling in 1951, suggested that most proteins have either an α-helix structure or a β-pleated sheet structure.
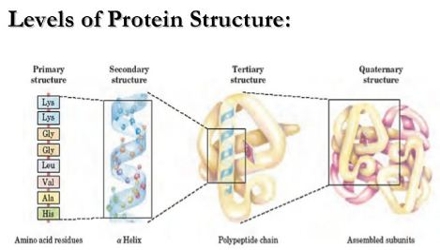
Structure of Protein:
It has three types of structures:
- Primary structure
- Secondary structure
- Tertiary structure
(a) Primary structure: The sequence in which amino acids are arranged in a polypeptide chain is called primary structure. Example: Human insulin.
(b) Secondary structure: The configuration which the polypeptide chain of a protein due to the secondary bonding such as hydrogen bonding between the carbonyl and amino groups is called secondary structure of the protein. The secondary structure shows corkscrew-like folding. Example: Hair protein, muscle protein, tendon, etc.
There are 3 types of secondary structures: α-helix, β-sheet and β-bond.
(c) Tertiary structure: Tertiary structure proteins arises due to folding and the super imposition of various secondary structural elements. Tertiary proteins are also called Glubular protein.