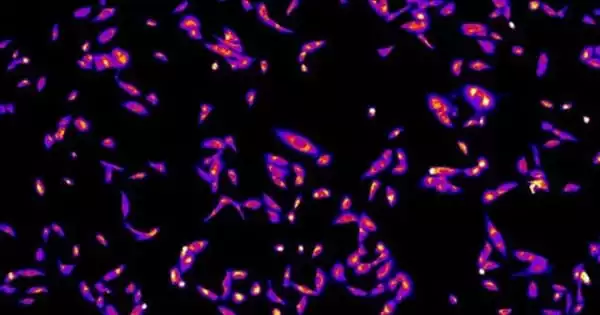
Researchers discovered that manipulating tumor cell voltage patterns using ion channel blockers that are already FDA-approved for other diseases can significantly reduce metastasis in animal models of breast cancer.
Electrical voltage patterns in normal cells serve as a blueprint for orderly growth. In the case of cancer, however, the opposite occurs. Because of a breakdown in the normal electrical patterns generated by the cells, they lose their specialized functions, begin to grow into a tumor, and spread into and disrupt the function of other tissues – metastasis. There are currently no clinically available treatments that specifically target the process of metastasis, which continues to be the leading cause of death in cancer patients.
Tufts University researchers have discovered that manipulating voltage patterns in tumor cells with ion channel blockers that are already FDA-approved for other diseases can significantly reduce tumor cell invasion in a dish and metastasis in an animal model of breast cancer. The discovery, published in eBioMedicine, demonstrated that drugs already approved for other conditions can slow or stop metastasis, potentially leading to a faster path to approval for cancer treatment.
“This is a relatively unknown, but highly opportunistic strategy for cancer treatment,” said Madeleine Oudin, Tiampo Family Assistant Professor of biomedical engineering at Tufts University School of Engineering and the study’s corresponding author. “Because ion channels, which regulate cell bioelectrical properties, are the second-most common target for existing pharmaceuticals, we have a relatively large set of ready-to-use drugs that could be repurposed for cancer therapy.”
Our findings suggest that a drug like amiodarone, which can change the bioelectric properties of cancer cells, could one day be combined with an existing chemotherapeutic drug that inhibits tumor cell growth to create a more effective cancer treatment.
Samantha Payne
The Tufts research team focused on triple-negative breast cancer (TNBC) to test their therapeutic strategy, a subtype of the disease that accounts for approximately 15% of all breast cancer cases. TNBC has a higher risk of metastasis than all other subtypes of breast cancer, and because it has a poor five-year prognosis, scientists are focusing their efforts on preventing it.
The researchers were able to demonstrate that manipulating the voltage properties of breast cancer cells has a significant effect on their progression to metastasis, reducing the number of metastatic sites in mouse lungs by approximately 50%.
Ion channels in the body actively push or passively allow positive and negative ions into and out of cells, resulting in a natural voltage across their membranes. Mike Levin, Vannevar Bush Professor in the Department of Biology and a collaborator on this study, has been studying the role of electrical properties in cellular behaviors in model organisms for many years. When Oudin joined Tufts, he was awarded funding through the Tufts Collaborates program, which supports collaborations between faculty from different schools to tackle new and exciting areas of research.

While a number of channels drive the movement of positively charged sodium, calcium, and potassium ions, as well as negatively charged chloride ions, potassium ion channels tend to dominate in generating voltage across the cell membrane. When Tufts researchers genetically overexpressed potassium ion channels in tumor cells, the inside of the cells became more negatively charged, and the voltage imbalance led to increased tumor growth and metastasis in both plated cells and animal models.
The researchers’ therapeutic strategy took the opposite approach: blocking potassium ion channels resulted in the cells regaining more normal voltages, decreased tumor cell invasion, and significantly reduced metastasis.
Four FDA-approved potassium ion channel blockers were tested, and they all had comparable efficacy in killing tumor cells. Amiodarone, the drug with the greatest effect on normalizing cell voltages, was chosen to see how well it would work in treating breast cancer in mice. The drug, which is approved for treating heart rhythm disorders, reduced the tumors’ ability to spread as cells broke off and moved to other parts of the body, according to the researchers.
They discovered a number of molecular pathways involved in cell movement by studying the genes that were activated by the voltage shift. The effects of the ion channel blocking drug were consistent with limiting cell movement so that new tumors do not form.
“Our amiodarone results were very exciting because they suggest that by targeting the bioelectric properties of tumor cells, we were able to specifically target the cells that leave the primary tumor, i.e., the harder to kill metastatic cells. Our findings suggest that a drug like amiodarone, which can change the bioelectric properties of cancer cells, could one day be combined with an existing chemotherapeutic drug that inhibits tumor cell growth to create a more effective cancer treatment” Samantha Payne, the study’s first author and a biomedical researcher in Oudin’s lab.
The Tufts team will continue to investigate the effects of ion channel blockers on cancer in animal models, as well as in combination with existing standard-of-care treatments such as chemotherapy. Because amiodarone and similar drugs are already approved for human use, phase I clinical trials in small groups of cancer patients could begin soon.