Scientists 3D printed human testicular cells and discovered promising early signs of sperm production. The researchers believe that one day the procedure will provide a cure for patients suffering from currently untreatable forms of male infertility.
UBC scientists have 3D printed human testicular cells and uncovered encouraging early evidence of sperm-producing capabilities in a pair of global firsts. The researchers, led by UBC urology assistant professor Dr. Ryan Flannigan, hope that the approach can one day provide a treatment for those suffering from currently incurable forms of male infertility.
After successfully 3D printing human testicular cells, scientists believe a treatment for male infertility is on the horizon. According to researchers at the University of British Columbia, these new cells show encouraging early signs of being able to create sperm, restoring men’s ability to father children.
“Infertility affects 15% of couples, and male factors are a contributing cause in at least half of those cases,” said Dr. Flannigan, whose lab is based at Vancouver General Hospital’s Vancouver Prostate Centre.
Infertility affects 15% of couples, and male factors are a contributing cause in at least half of those cases. We’re 3D printing these cells into a very precise form that matches human anatomy, which we believe will give us the best chance of triggering sperm production.
Dr. Flannigan
“We’re 3D printing these cells into a very precise form that matches human anatomy, which we believe will give us the best chance of triggering sperm production. If effective, this could pave the way for novel reproductive therapies for couples who don’t have any other options right now.”
Sperm is produced in the human testicles by microscopic tubes known as seminiferous tubules. Due to decreased sperm production within these structures, no sperm is discovered in ejaculate in the most severe form of male infertility, known as non-obstructive azoospermia (NOA).
While doctors can help NOA patients in some situations by undergoing surgery to find highly uncommon sperm, Dr. Flannigan claims that this operation is only successful about half of the time. “Unfortunately, the other half of these people have no options because we can’t obtain sperm for them.” These are the patients Dr. Flannigan’s team hopes to assist.
The researchers performed a biopsy to obtain stem cells from the testicles of a NOA patient for the recent study. The cells were then cultivated and 3D printed onto a petri dish to form a hollow tubular structure resembling sperm-producing seminiferous tubules.
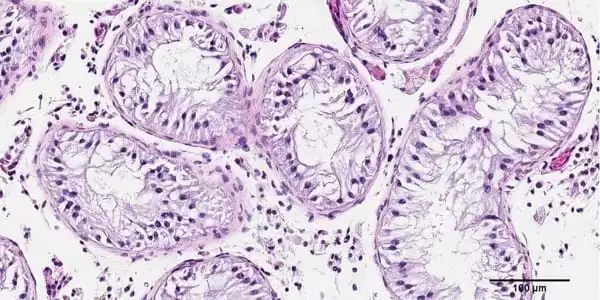
The team discovered that the cells had survived 12 days after printing. Not only that, but they had matured into several of the specialized cells involved in sperm production and were demonstrating a significant improvement in spermatogonial stem cell maintenance – both of which were early indicators of sperm generating potential. The study’s findings were just published in Fertility and Sterility Science.
“Seeing these cells survive and begin to differentiate is a big accomplishment. There is a long road ahead of us, but this gives our team a lot of hope “Dr. Flannigan stated.
The researchers are now attempting to “train” the printed cells into making sperm. To accomplish this, they will expose the cells to various nutrients and growth stimulants, as well as fine-tune the structural arrangement to allow for cell-to-cell interaction. If they can get the cells to create sperm, the sperm might be utilized to fertilize an egg via in vitro fertilization, giving couples a new reproductive therapy option.
Dr. Flannigan’s research program has also offered new information on the genetic and molecular pathways underlying NOA. They’ve been employing multiple single cell sequencing techniques to understand the gene expression and features of each individual cell, then using computer modeling of this data to better understand the condition’s core causes and propose novel therapy possibilities. The study has been very collaborative, with UBC researchers from computer science, mathematics, and engineering participating, as well as foreign collaborations.
“We’re discovering more and more that there are likely many distinct causes of infertility, and that each case is very patient specific,” Dr. Flannigan said. “With that in mind, we’re taking a personalized, precision medicine approach: we take cells from a patient, try to identify what abnormalities are specific to them, and then 3D print and maintain the cells in ways that overcome those original shortcomings.”