Spectroscopy and electromagnetic spectra
Spectroscopy is the study of how light interacts with matter. We can use spectroscopy to determine the structure and functional groups in organic compounds. It is a technique that uses the interaction of energy with a sample to perform an analysis.
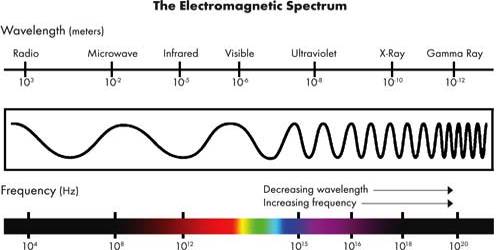
Electromagnetic spectra is the entire range of wavelengths or frequencies of electromagnetic radiation extending from gamma rays to the longest radio waves and including visible light
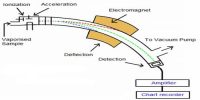
Infrared, ultraviolet, and nuclear magnetic resonance spectroscopy involves the interaction of molecules/atoms with electromagnetic radiation and thus differ from mass spectrometry where high energy electron beam is used to ionize the sample particles.
Visible light, infrared and ultraviolet (UV) radiation, X-rays, microwaves, radio waves are all different kinds of electromagnetic radiation. The wavelengths of the different kinds of radiation are shown in Figure.
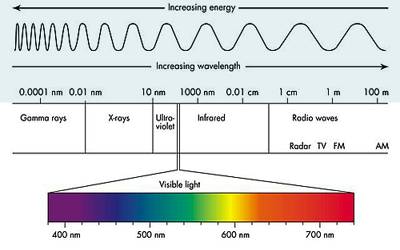
When electromagnetic radiation falls on a sample, part of the radiation may be absorbed, a part may go through (transmitted) or diffracted. Our interest will be on the part of the radiation that is absorbed by the sample.