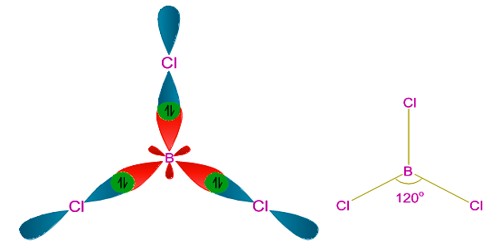
sp2 hybridization occurs in Boron Trichloride BCl3 molecule.
The hybridization in boron trichloride is sp2 hybridization. This accounts for the aptitude of boron to bond to three other atoms and has trigonal planar molecular geometry when it only has one partially-filled p-orbital capable to make a bond.
sp2 hybridization: When one s and two p orbitals of an atom mix themselves and produce three equivalent orbitals, the process is called sp2 hybridization. The three hybrid orbitals produced in sp2 hybridization lie on a plane and make 1200 to each other.
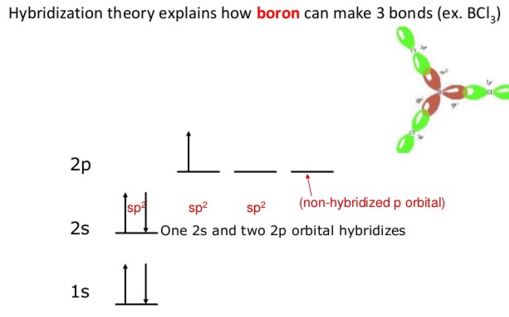
Ground state pattern being 1s2 2s2 2p1, it has only one unpaired electron. Since three unassociated electrons are necessary so there is support of one of 2s electron into the 2p sublevel by gripping energy.
Thus the pattern becomes 1s2 2s2 2px1 2py1.
However, to account for the trigonal planar shape of this BCl3 molecule, sp2 hybridization before the bond structure was advocated.
Boron experiences sp2 hybridization by using a 2s and two 2p orbitals in the excitation state to give three half-filled sp2 hybrid orbitals which are oriented in trigonal planar equilibrium. It then forms three σsp-p bonds with three chlorine atoms by using its half-filled sp2 hybrid orbitals. For the σ-bond formation, the half-filled p-orbital are used.