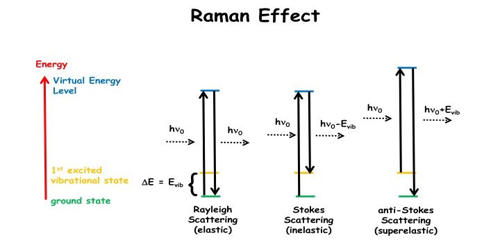
In 1928, Sir C.V. Raman discovered experimentally, that the monochromatic light is scattered when it is allowed to pass through a substance. The scattered light contains some additional frequencies other than that of incident frequency. This is known as the Raman effect.
Raman effect, change in the wavelength of light that occurs when a light beam is deflected by molecules.
Raman spectroscopy is used in many varied fields – in fact, any application where non-destructive, microscopic, chemical analysis and imaging is required. When a beam of light traverses a dust-free, transparent sample of a chemical compound, a small fraction of the light emerges in directions other than that of the incident (incoming) beam. Whether the goal is qualitative or quantitative data, Raman analysis can provide key information easily and quickly. The spontaneous Raman effect takes place when the molecules are excited from the ground state to a virtual state and relax into a vibrationally excited state forming Stokes Raman scattering.
Raman effect is the inelastic scattering of a photon by molecules which are excited to higher vibrational or rotational energy levels. Raman effect takes place when light enters a molecule and interacts with the electron density of the chemical bond causing electromagnetic field in the molecule leading to vibrational and deformation of frequency shift. Molecules absorb the energy of the incident radiation, just to re-emit photons of frequencies different from that of the incident ray. It can be used to rapidly characterize the chemical composition and structure of a sample, whether solid, liquid, gas, gel, slurry, or powder.