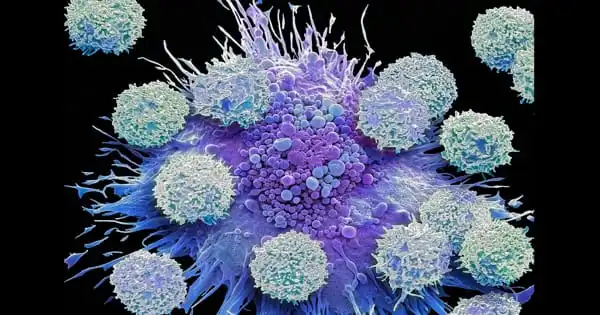
Wound healing is the process by which a live entity replaces destroyed or damaged tissue with newly generated tissue. Scientists have discovered that during wound healing, the recycling activity of cells, autophagy, leads to the fusing of multiple single cells into multinucleated cell units.
A study led by Maria Leptin discovered that autophagy, a stress response process in cells, plays a crucial role in wound healing in the fruit fly Drosophila: When a wound heals, the protein complex TORC1 initiates and regulates the autophagy process. This is a recently found autophagy function and the first evidence that autophagy regulates the development of syncytia (multinucleated cells).
While syncytia are generated during the development of muscles or the placenta, their importance in wound healing and the participation of autophagy are novel discoveries. The research, ‘Autophagy-mediated plasma membrane clearance enhances the development of epithelial syncytia,’ was published in The EMBO Journal.
When a wound heals, the protein complex TORC1 initiates and regulates the autophagy process. This is a recently found autophagy function and the first evidence that autophagy regulates the development of syncytia.
Maria Leptin
Autophagy is a cellular recycling mechanism that has been passed down from yeast to humans. Autophagic vesicles, which are microscopic bubbles within the cell, recognize, engulf, and digest intruders such as bacteria and viruses as well as cell debris such as accumulated protein clumps. The material is recycled, supplying raw resources to the cell under demanding conditions.
When correct cellular function degrades and toxic products accumulate in organs due to aging, illness, or disease, autophagy activity plays an important role in restoring health. Autophagy malfunction, in turn, raises the risk of neurological illnesses like Alzheimer’s and Parkinson’s, as well as cancer and infections.
The CECAD Cluster of Excellence for Aging Research, the Center for Molecular Medicine Cologne (CMMC), and the Institute of Genetics (all at the University of Cologne) investigated the role of autophagy in wound healing and the healthy and uninjured epidermis (skin) of the fruit fly Drosophila melanogaster in the new study. Thus, in the process investigated here, autophagy did not digest viruses or bacteria, but rather the cell’s own cell membrane, resulting in the breakdown of cell borders and the formation of a giant cell with numerous nuclei.
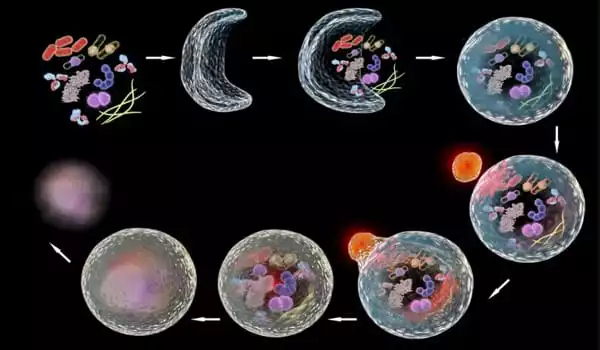
The researchers discovered that autophagy is elevated in the cells surrounding the wound. As a result, the membranes that connect the cells are selectively broken down. A huge, multinucleated cell (syncytium) eventually forms. ‘When we genetically induced autophagy in healthy, uninjured skin, we saw the same thing: the membrane between adjacent cells is destroyed, and vast patches of multinucleated syncytia appear throughout the epidermis,’ said Parisa Kakanj, the study’s first author.
‘It is remarkable that syncytia development, which we already know occurs in the formation of some organs such as muscles or the placenta, also occurs in wound healing. Because multinuclear cells are common in tumors and diseased organs, the involvement of autophagy in this may be crucial for our knowledge of disease mechanisms.’
Previous research on muscle and placenta development found that the establishment of a syncytium provides mechanical stability as well as a strong barrier function to protect tissues from infections. It is unclear whether the mechanism serves similar activities in wound healing. This will be the focus of future research.
The investigators also observed an interaction of the autophagic vesicles with the lateral plasma membrane of the cell in the healthy, unwounded epidermis as well as in the cells surrounding the wound. In this context, proper function of the TORC1 protein complex, which has a central regulatory function in cell metabolism, and here also controls autophagy, is crucial to prevent destruction of the epidermis by autophagy.
Based on these and other findings, the team hypothesizes that the lateral plasma membrane is a possible source of autophagic vesicles. ‘Autophagy is a two-edged sword, the precise quantity and spatiotemporal activity of which determines whether it is useful or detrimental,’ explained Maria Leptin. To be able to harness autophagy’s favorable side for prevention and therapy, it is even more crucial to understand how autophagy is activated and autophagic vesicles are generated.