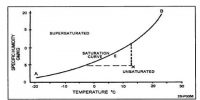
Effect of Temperature on Solubility
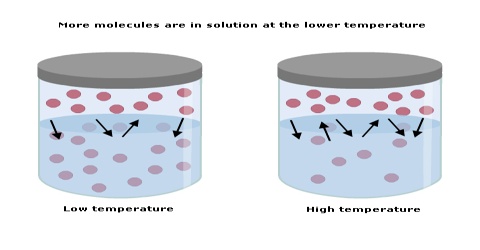
The solubility of a gas in a liquid is markedly affected by temperature. Usually, the solubility decreases with the increase of temperature. This can be seen from the data in below table. It can be readily shown that the variation of solubility with temperature follows a general pattern. Considering solubility expressed as concentration as an equilibrium constant, for gas-liquid systems the relation between concentration and temperature assumes the form
(d ln C) / dT = ∆H/RT2
or, ln C2/C1 = – ∆H/ RT2 [(1/T2) – (1/T1)]
where C1 and C2 are the concentrations in mol L-1 temperature T1 and T2 and ∆H is the enthalpy of solution of 1 mole of the gas in the saturated solution.
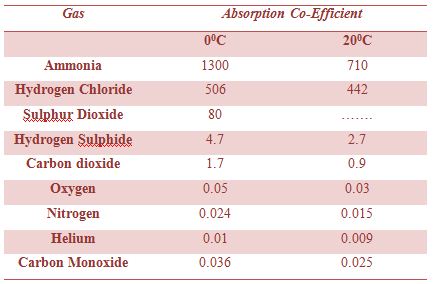
Table: Absorption co-efficient of some gas
Theoretically all gases should be completely expelled from a solution at the boiling point of the liquid since at the boding point the vapour pressure of the liquid is equal so the superincumbent pressure. This is obeyed by many solutions, but the removal of the last trace at the gas is sometimes difficult and takes a long time. In removing gases from solution this fact should be borne in mind. This m the Kjeldhal method for estimation of Nitrogen the ammonia solution formed in an intermediate step is boiled for a sufficiently long time to expel all ammonia.
Some factors that affect the solubility of a substance are temperature, air pressure and the type of solvent used. The solubility of different substances will not be the same for a given solvent. For example, some substances can dissolve very easily in water whereas others may not. Experimentally it is found that the solubility of most compounds depends strongly on temperature and, if a gas, on pressure as well. As we shall see, the ability to manipulate the solubility by changing the temperature and pressure has several important consequences.