Obesity is a nationwide phenomenon. Moreover, the related health issues have a major economic effect on the US health care sector, spending $147 billion a year. In addition, obesity places people at risk for chronic illnesses such as diabetes, respiratory disease, and certain cancers. For people with a body mass index higher than 35 or who have at least two obese-related disorders, surgery provides a means for patients not only to shed extra weight but to control their weight over the long term.
In a new study, researchers have described a medical device that might help with weight loss and requires a simpler operative procedure for implantation.
Gastric bypass surgery is often the final resort for people dealing with obesity or who have severe health complications due to their weight. As this operation includes constructing a narrow abdominal pouch and rerouting the digestive tract, it is very invasive and prolongs the healing time for patients. In a recent study, researchers at Texas A&M University identified a medical device that could assist with weight loss and allows a simplified surgical technique for implantation.
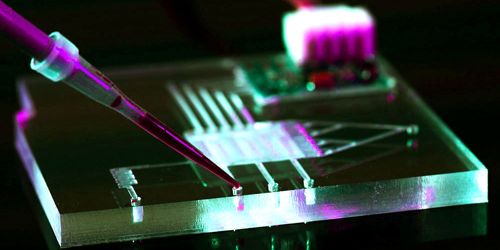
Researchers said that their centimeter-sized unit offers a sense of fullness by activating the ends of the vagus nerve with light. Unlike other systems requiring a power cable, their computer is wireless and can be operated externally from a remote radio frequency source.
Tiny wireless device sheds light on combating obesity
“We wanted to create a device that not only requires minimal surgery for implantation but also allows us to stimulate specific nerve endings in the stomach,” said Dr. Sung II Park, assistant professor in the Department of Electrical and Computer Engineering. “Our device has the potential to do both of these things in the harsh gastric conditions, which, in the future, can be hugely beneficial to people needing dramatic weight-loss surgeries.”
Further details about their device are published in the January issue of Nature Communications.
In recent years, the vagus nerve has gained a great deal of focus as a priority for the treatment of obesity, since it sends sensory knowledge about the fullness of the stomach lining to the brain. While there are medical devices that can trigger the vagus nerve endings and thus help to relieve hunger, these devices are similar in nature to the pacemaker, i.e. wires attached to the current source give electrical jolts to trigger the tips of the nerve.
However, Park said that cellular technologies, as well as the use of innovative genetic and optical techniques, have the ability to make nerve stimulation systems less cumbersome and more convenient for the user. “Despite the clinical benefit of having a wireless system, no device, as of yet, has the capability to do chronic and durable cell-type specific manipulation of neuron activity inside of any other organ other than the brain,” he said.
To fix this void, Park and his team first used genetic techniques to express genes that respond to light into particular in vivo vagus nerve endings. Then they built a small, paddle-shaped gadget and placed micro LEDs at the tip of its flexible shaft, which was connected to the stomach. The microchips used for the computer to interact wirelessly with an external radio frequency source were located in the head of the device called the harvester. The harvester was also designed to generate small currents to control the LEDs. When the radio frequency source was turned on, the researchers found that the LED light was effective in suppressing hunger.
The researchers said they were shocked to find that the biological devices coordinating the elimination of hunger in their experiments were distinct from traditional wisdom. In other words, it is commonly known that when the stomach is complete, it stretches and stretch information is transmitted to the brain by mechanor receptors on the vagus nerve.
“Our findings suggest that stimulating the non-stretch receptors, the ones that respond to chemicals in the food, could also give the feeling of satiety even when the stomach was not distended,” said Park.
Looking forward, he said that the new system could also be used to control nerve ends in the gastrointestinal tract and other tissues, such as the intestine, with few to no adjustments. “Wireless optogenetics and identifying peripheral neural pathways that control appetite and other behaviors are all of great interest to researchers in both the applied and basic fields of study in electronics, material science, and neuroscience,” Park said. “Our novel tool now enables interrogation of neuronal function in the peripheral nervous systems in a way that was impossible with existing approaches.”