Adiabatic change
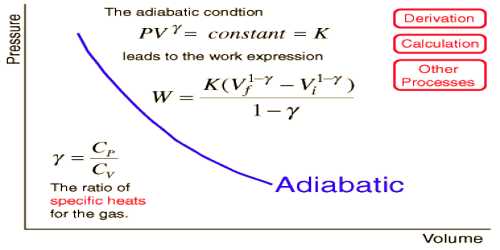
When a gas is suddenly compressed then an amount of heat is produced. If this heat is not removed, then the temperature of the gas will increase. Again when a gas is suddenly allowed to expand, the gas will lose some amount of heat, and if an equal amount of heat is not supplied from outside, the temperature will decrease. So, in this change temperature never remains constant. Furthermore, it is mentioned that in this case the gas does neither receive heat nor reject heat, but internal energy of the gas does not remain constant – increase or decrease of internal energy takes place. This type of change is called adiabatic change, ‘a’ means ‘no’ and ‘dia’ means ‘through’ and ‘bates’ means ‘heat’. In a single word ‘adiabatic’ means ‘heat not passing through’. That means heat neither enters into the system nor the system rejects heat. In case of adiabatic change the equations, PVϒ = constant and TVϒ-1 = constant is applicable.
The process in which a system neither receives heat nor rejects heat is called adiabatic process. The change in which no heat is supplied from outside or heat is not ejected from the gas, but the change of pressure and volume takes place, is called adiabatic change.
Or, The process during which change of pressure and volume takes place but no change of heat takes place, i.e., the system (here gas under process) does not accept or reject heat, but the change of temperature takes place is called adiabatic process. This change is called adiabatic change.
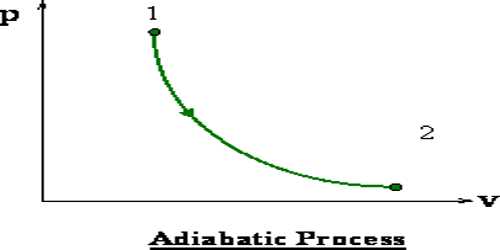
In case of adiabatic change, Boyle’s law is not applicable. In this case, the relation between pressure and volume is PVϒ = contestant and relation between temperature volume is TVϒ-1 constant. In case of adiabatic change P and V curve is called the adiabatic curve. In the figure, an adiabatic curve has been shown. The adiabatic curve is comparatively steeper than the isothermal curve.