Aluminium isopropoxide is a colorless, volatile liquid that is soluble in non-polar solvents such as toluene and hexane. It is the chemical compound usually described with the formula Al(O-i-Pr)3, where i-Pr is the isopropyl group (–CH(CH3)2). This colorless solid is a useful reagent in organic synthesis.
It is an organometallic compound that is commonly used as a precursor for the synthesis of various aluminium-containing materials, including ceramics, coatings, and catalysts.
Properties
Aluminium isopropoxide is a colorless to pale yellow liquid with a pungent odor. It is soluble in organic solvents, such as ethanol, ether, and benzene, but insoluble in water. It is a highly reactive compound that is sensitive to moisture, air, and acids.
- Chemical formula: C9H21AlO3
- Molar mass: 204.246 g·mol−1
- Appearance: white solid
- Density: 1.035 g cm−3, solid
- Melting point: [Sensitive to purity: 138–142 °C (99.99+%); 118 °C (98+%)]
- Boiling point: @10 Torr 135 °C (408 K)
- Solubility in water: Decomposes
- Solubility in isopropanol: Poor
- Crystal structure: monoclinic
Structure
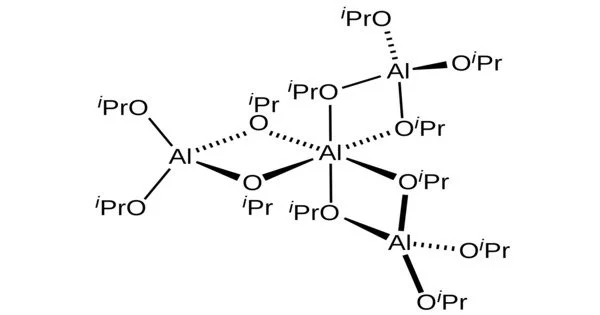
A tetrameric structure of the crystalline material was verified by NMR spectroscopy and X-ray crystallography. The species is described by the formula Al[(μ-O-i-Pr)2Al(O-i-Pr)2]3. The unique central Al is octahedral, and three other Al centers adopt tetrahedral geometry. The idealised point group symmetry is D3.
Preparation
Aluminium isopropoxide is prepared by the reaction of aluminium metal with isopropyl alcohol. The reaction is exothermic and typically carried out under inert gas conditions to prevent the formation of unwanted by-products.
This compound is commercially available. Industrially, it is prepared by the reaction between isopropyl alcohol and aluminium metal, or aluminium trichloride:
2 Al + 6 iPrOH → 2 Al(O-i-Pr)3 +3H2
AlCl3 + 3 iPrOH → Al(O-i-Pr)3 + 3 HCl
The process involves heating a mixture of aluminum, isopropyl alcohol, and a trace of mercuric chloride. The process involves the formation of an aluminum amalgam. To start the reaction, a catalytic amount of iodine is sometimes added. Mercury is not used in the industrial route.
Reactions
Aluminium isopropoxide is used in MPV reductions of ketones and aldehydes, as well as secondary alcohol oxidation by Oppenauer. It is assumed that the tetrameric cluster disaggregates in these reactions. It is a component of the Tishchenko reaction.
Application
Some of the applications of aluminium isopropoxide include the synthesis of aluminum oxide films and nanoparticles, the preparation of alumina-supported catalysts, and the production of ceramic materials. It is also used as a crosslinking agent for organic polymers and as a curing agent for epoxy resins.
It is important to handle aluminium isopropoxide with care, as it is flammable and can react violently with water, acids, and other reactive substances. Proper protective equipment and procedures should be used when handling this compound.