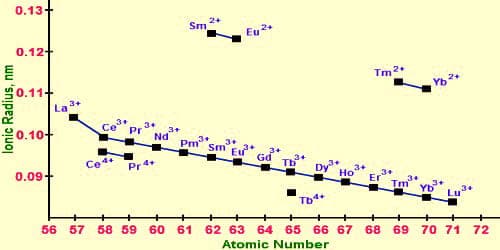
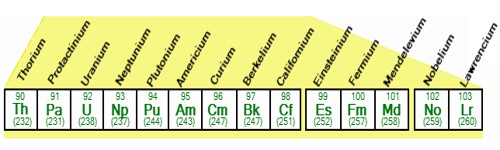
The cause of actinide contraction is the imperfect shielding by 5f-electrons. As we proceed from one element to the next one in the actinide series, the nuclear charge increases by +1 at each next element which is not compensated due to poor shielding effect of 5f orbitals due to their more diffuse shape. Hence as the atomic number increases, the inward pull experienced by 5f-electrons increases. The elements with atomic numbers 90 to 103, i.e., thorium to lawrencium (which come immediately after actinium, Z = 89) are called actinides.
The regular reduce in the ionic radii with the raise in atomic no. is called actinide contraction. It is due to the deficient protecting of one 5f electron by another in a similar subshell. In actinide elements, the differentiating electrons entered their inner 5f orbitals. Due to the deprived screening effect of 5f electrons Zeff on outer electrons increases. Hence as the atomic number increases, the inner pull experienced by 5f-electrons increases. When one electron is added to the 5f-orbital every time means proton keeps on increasing due to which the nuclear charge increases erratically which causes the deprived shielding consequence.
The gradual decrease in the ionic radii with the increase in atomic no. is called actinoid contraction. Consequently, a steady decrease in size occurs in the actinide series. This type of contraction also occurs in actinides. The jump in contraction between the consecutive elements in the actinides is greater than lanthanides. This is due to the lesser shielding of 5f−electrons which are therefore pulled more strongly by the nucleus.
As a result of which the size of atoms or ions usually decreases. This steady reduction in the size with a growing atomic number is called actinide reduction like lanthanide contraction. As a result, a balanced reduce in size occurs in the actinide series. Because of the very deprived defensive by 5f electrons, a reduction is larger along the period.