Group 18 of the periodic table consists of helium, neon, argon, krypton, xenon and radon. All these are gases under ordinary conditions of temperature and pressure. All of them (except Rn) are present in the air in traces. Rn is obtained from the radioactive disintegration of radium.
On account of their very minute quantities in the atmosphere, they were named as rare gases. Due to their chemical inactivity, these were named as inert gases. A number of xenon compounds and two krypton fluorides were prepared and thus they were named as noble gases.
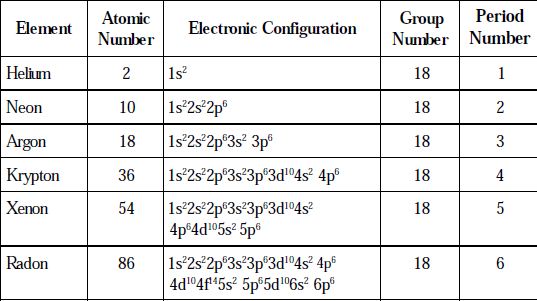
Electronic Configuration
All these elements possess ns2 np6 configuration. The differentiating electrons enter into the p-subshell and thus are included in p-block elements.
Table: Electronic Configuration of Group 18 elements