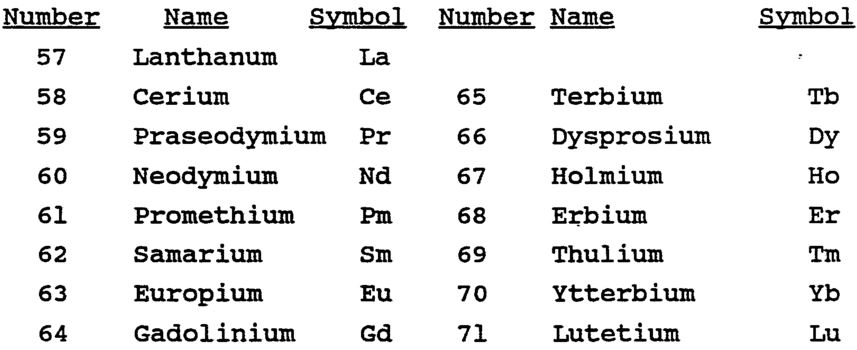
The Lanthanide series include fifteen elements i.e. lanthanum (57La) to lutetium (71Lu). Lanthanum and Lutetium have no partly filled 4f- subshell but have electrons in 5d-subshell. Thus these elements should not be included in this series. However, all these elements closely resemble lanthanum and hence are considered together.