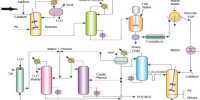
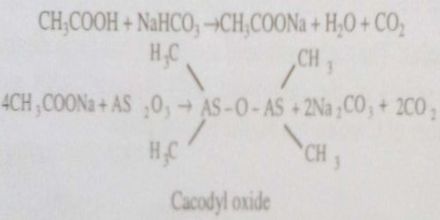
Acetic acid is treated with sodium bicarbonate till effervescence is ceased. The solution thus obtained is evaporated to dryness when a solid residue is obtained. The solid residue is treated with finely powdered arsenious oxide and the mixture is heated, then vapour of cacodyl oxide having a very offensive smell is given out.
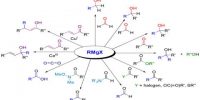
A reaction of acetic acid with an alcohol will produce an ester (typically, sweet smelling). E.g., reaction with ethyl alcohol (with sulfuric acid present as a catalyst and a desiccant to remove water produced) produces ethyl acetate, which is commonly found in nail polish remover and has a fruity smell.