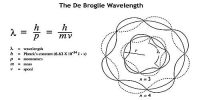
The position and the velocity of the bodies which we come across in our daily life can be determined accurately at a particular instant of time. Hence the path or trajectories of such bodies can be predicted.
However, Werner Heisenberg in 1927 pointed out that we can never measure simultaneously and accurately both the position and velocity (or momentum) of a microscopic particle as small as an electron. Thus, it is not possible to talk of trajectory of an electron. This principle, which is a direct consequence of the dual nature of matter and radiation, states that,
“it is impossible to measure simultaneously both the position and velocity (or momentum) of a microscopic particle with absolute accuracy or certainty.”
Mathematically, uncertainty principle can be put as follows.
∆x.∆p ≥ h/4π
where, Δx = uncertainty in the position of the particle and
Δp = uncertainty in the momentum of the particle.
The sign ≥ means that the product of Δx and Δp can be either greater than or equal to h/4π but can never be less than h/4π.