Though closely related, heat and temperature do not bear same meaning. There is a great difference between them. The differences are stated below:
- Heat is a form of energy but temperature represents a thermal condition of the body.
- Heat is the cause and temperature is the effect.
- When a body absorbs heat it’s temperature increases and when the body gives up heat it’s temperature decreases.
- Two bodies at the same temperature may have different amount of heat. Similarly two bodies containing equal amount of heat does not equal in temperature.
- Transfer of heat from one body to another body depends on the temerature difference between the bodies.
- The instrument for meassuring heat is called calorimeter and the instrument for measuring temperature is called thermometer.
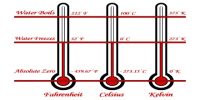
- Unit of heat is Joule and the unit of temperature is oC or K or oF.
- Heat is energy and temperature is the demonstration of energy.
- Heat flows from a body of higher temperature to a body of lower temperature.
- Heat is proportional to the total energy of the molecules in the body; but temperature is proportional to the average kinetic energy of molecule of the body.
- The branch of physics which measures heat is called calorimetry and the branch of physics which measures temperature is called thermometry.
- Temperature does not have dimension but dimension of heat is the dimension of energy [ML2T-2].