Excitation and Ionization Potential of an Atom
Excitation is the addition of a discrete amount of energy to a system such as an atomic nucleus, an atom, or a molecule. It is the result of energy being given to an electron moving it to a higher energy level. Ionization potential is the amount of energy required to remove the most loosely bound electron from a neutral, gaseous atom. If enough energy is given to the electron to remove it from the atom ionization has occurred.
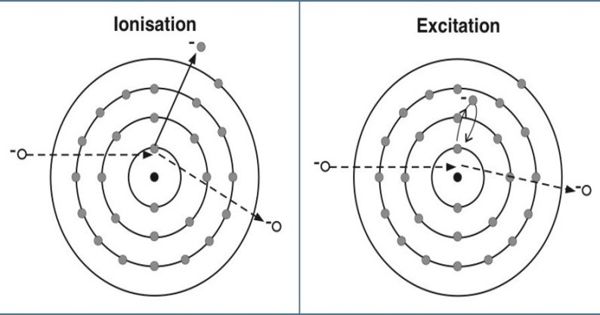
According to Bohr’s theory, there are certain discrete orbits permitted for the motion of the electron. An electron can revolve in these orbits without radiating energy. An atom is said to be in the ground state when its energy is least. Before an atom can emit spectral radiation, the electron in it has to be raised to a higher orbit. This process is known as the excitation of the atom. The energy required to raise an atom from its normal state into an excited state is called the excitation potential energy of the atom. If the energy supplied to an electron is such that the electron is lifted from its ground state to one of the higher allowed orbits, the atom will be excited and the energy supplied is called excitation energy or excitation potential. For example, the energy required to transfer the electron in a hydrogen atom from the ground state to the first excited state = (13.6-3.4) = 10.2eV. The energy required to raise it to the second excited state = (13.6 – 1.51) = 12.09 eV. The potentials corresponding to these energies are called as the excitation potentials.
Excitation explains the movement of an electron from a lower energy level to a higher energy level. Ionization potential explains the removal of an electron from an energy level completely.
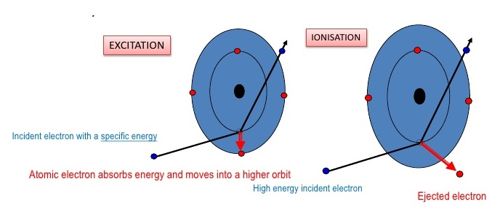
The ionization potential is that accelerating potential which makes the impinging electron acquires sufficient energy to knock out an electron from the atom and thereby ionize the atom. If the energy supplied to an electron is such that the electron is lifted from its ground state to an orbit at infinity, the atom is said ion ionized, and the energy supplied is called the ionization energy or ionization potential. For a hydrogen atom, the energy required to remove an electron from the first orbit to its outermost orbit (n=∞) 13.6-0=13.6eV. This energy is known as the ionization potential energy for a hydrogen atom. 13.6 V is the ionization potential of a hydrogen atom. The energy is so much that the electron disengages it from the atom but its kinetic energy outside the atom is zero.
The excitation potential and ionization potential are called the critical potentials of the atom. Excitation and ionization potential in chemistry are two terms used to explain the relationship between energy changes and the atomic behavior of chemical elements. The critical potential of an atom is defined as the minimum potential required to excite a free neutral atom from its ground state to a higher state.