Task: To make pure salt from impure salt.
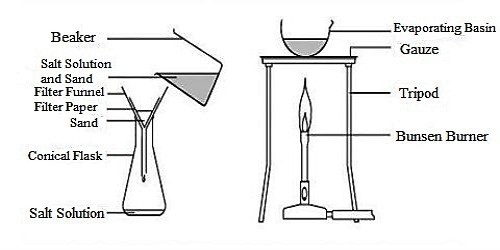
Required accessories: The heterogeneous mixture of impure salt, stirrer, tripod stand, wire-net, funnel, filter paper, stand with the ring, water.
Procedure: At first take a filter paper and fold it into four equal parts. Next set it in the funnel keeping three folds at one side and one fold at the other side as shown in the figure. Soak the filter paper slightly with water so that the filter papers do not get displaced. Place the funnel in the ring attached to the stand as shown in the figure. Keep a beaker under the funnel. Now, pour the heterogeneous mixture of impure salt (taking from the previous experiment) slowly on the filter paper. Wait until all the clean water drains out of the funnel.
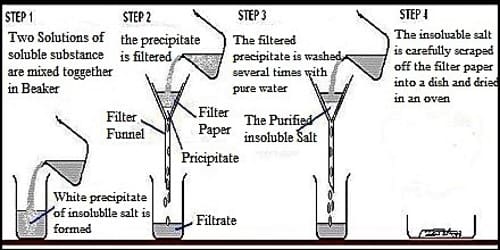
Fig: Make Pure Salt from Impure Salt procedure
Now dry up the entire water by placing a beaker of pure saline water on the wire net over the tripod stand and applying heat at the bottom of the beaker with the help of burning spirit lamp.
Analysis:
What happened after pouring the mixture in the funnel? The impurity (soil particles) free pure saline water slowly passes through the filter paper and is collected in the beaker under the funnel. The impurities (particles) of soil were detained by the filter paper. This process of separating the panicles of soil from the mixture of soil and water by the filter paper is called filtration. Therefore, filtration is such a process by which solid substances can be separated from the heterogeneous mixture of solid and liquid.
After becoming the entire water of the beaker quite dry, what do you obtain? You obtain layers of white, dazzling and clean salt. Because all the impurities of the impure salt taken at the beginning were entirely eliminated by filtration with a filter paper.
Getting salt from rock salt – How do we acquire pure salt from rock salt?
It is often necessary to obtain pure solid chemicals from impure samples. If students have regularly carried out this type of experiment, e.g. salt from a combination of salt and sand, it can be presented as an easy form of investigation.
- Crush some rock salt using the mortar and pestle.
- Put the crushed rock salt into a beaker and add some water. Stir the mixture.
- Suspiciously filter the mixture. The liquid that comes through the filter paper should be clear.
- Put a little liquid in an evaporating basin and heat it. Wear eye guard.
- The powder that is left in the evaporating basin should be pure salt.
Procedure:
Step 1: Rock salt is compressed into little pieces and added to water. The salt dissolves in the water but the unsophisticated impurities are insoluble.
Step 2: The solid sandy bits are separated from the salt solution using filtration. The combination is passed through some filter paper held in a funnel. The salt solution can bypass throughout the small holes in the filter paper because all the particles are tiny. The unsophisticated impurities can’t pass throughout the holes, because the particles are too big.
Step 3: The salt solution is distorted to salt by evaporating the water. The solution is heated so that the water evaporates, leaving the salt behind.
Filtration is often used to separate the solid and liquid parts of a suspension. The liquid part is called the filtrate. The solid that remains on the filter paper is called the filtrate.
Crystallization of salt is defined as the procedure in which we dissolve the salt in a solvent. Then some of its particles will dissolve and some will continue undisclosed which are the impurities.
Thus, we can conclude that the method that is used to get pure salt from impure salt is crystallization.