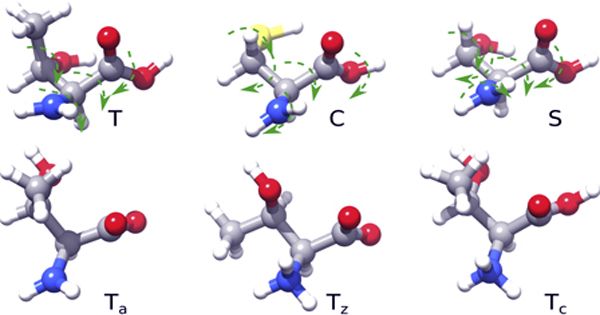
For a detailed analysis of a chemical system, it is necessary to know all the relevant intermediates and initial reactions of the potential energy surface (PES) attached to them. Deep-perception of all response lessons is lost to study the evolution of a system over a given period of time with a set of initial conditions and suggest derivatives of the original reactions to avoid unwanted side effects. Manual probes of complex reaction methods employed in quantum-chemical methods are slow and error-prone. Furthermore, due to the high dimensions of the PES, a complete search is generally unbearable. However, the formation of unwanted side products or digestive reactions needs to be logically exposed to unexpected reactions.
Complex reaction processes are ubiquitous in chemistry. They are, for example, the basis of a transition-metallic catalyst, Polymerization, cell metabolism, enzyme catalysts, and Environmental processes and systems is the goal of chemistry. To know all the chemical compounds and the initial reactions of a particular chemical process, it is necessary to understand the nuclear details. Although many chemical reactions result in a major product picking generation, in general, multiple reaction pathways compete with each other. Reactive species (e.g., radicals, valence-unsaturated species, charged reactants, strong acids, and bases, etc.) or light, advanced temperatures, or pressure increases through high-energy (electronic or vibrating) states.
For the investigation of chemical reaction networks, the identification of all relevant mediators and primary reactions is mandatory. There are many algorithmic methods that perform explorations efficiently and in an automated fashion. These methods differ in the range of their application, the degree of completeness of the investigation, and the amount of heuristics and human intervention required. Here, we describe and compare different approaches based on these criteria. Chemical heuristics, human interaction, and the strengthening of physical rigidity are discussed in future directions.
Chemical reaction process, the sequence of primary chemical reactions that combine sets of reactants and product molecules occupy a central position in synthesis, catalysts, and biochemistry. The characteristic of a reaction method is often fundamental to changes in technological steps, for example, a better understanding of chemical processes related to the emergence of antibacterial resistance in microbes. In principle, the next generation of antibiotics could develop, while mechanical insights could also help the diaphragm, and ultimately the catalyzed organ metallic- complex of biological transformations. maybe used to synthesize these new drugs such, the investigation of chemical reaction mechanisms remains a perennial challenge for both experimental and reputable chemists.