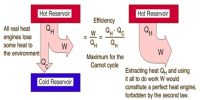
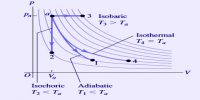
Thermodynamics is the science of the relationship between heat and other forms of energy. Thermochemistry is the area of Thermodynamics related to the heat changes involved in chemical reactions.
Forms of Energy: Energy is defined as the capacity to move matter. The units of energy are Joules (J). Energy can be in many forms:
- Radiant Energy such as Light,
- Thermal Energy such as Heat,
- Energy stored within a compressed spring, and
- Sound Energy.
There are three main types of energy:
- Kinetic Energy is the energy associated with the movement of an object,
- Potential Energy is the stored energy an object has because of its position (e.g. gravitational, magnetic or electrostatic),
- Internal Energy is the sum of the kinetic and potential energies of the particles making up a substance.
Kinetic Energy: An object of mass m and speed or velocity v has a kinetic energy Ek equals to 0.5 m v2.
Potential Energy: This energy depends on the ‘position” (such as height) in a “field of force” (such as gravity or the compression of a spring). For example, a ball of a given mass m at the top of a mountain is at a relatively high “position” h in the “gravitational field” g of the earth. Its potential energy Ep is given by Ep = m g h where m = mass, g = gravity and h = height.
Potential energy is not just “height” energy. Other situations where energy is stored include:
- A compressed spring,
- Two magnets held near together, and
- Charged particles near together.
Internal Energy: The internal energy is the energy of the particles (the electrons, protons and neutrons) making up a substance. The internal energy includes the kinetic energy of the electrons and nuclei; and the potential energy related to the electronic energy levels, electrostatic attractions etc.