Hydrocarbons and petroleum are almost synonymous with environmental science. After all, oil deposits pay for almost all the hydrocarbons we encounter. However, the few hydrocarbons that trace their origin to biological sources may play a greater ecological role than was initially assumed by scientists. A team of researchers at UC Santa Barbara and Woods Hole Oceanographic Institution explored this historically ignored field of oceanography for evidence of a neglected global cycle. They also tested how its presence could have an effect on the ocean’s reaction to oil spills.
“We have shown that there is a large and rapid hydrocarbon cycle in the ocean and that it is distinct from the ocean’s ability to react to oil inputs,” said Professor David Valentine (link is external), who holds the Norris Chair of the Department of Earth Sciences at UCSB.
A team of researchers at UC Santa Barbara and Woods Hole Oceanographic Institution investigated this previously neglected area of oceanography for signs of an overlooked global cycle. They also tested how its existence might impact the ocean’s response to oil spills.
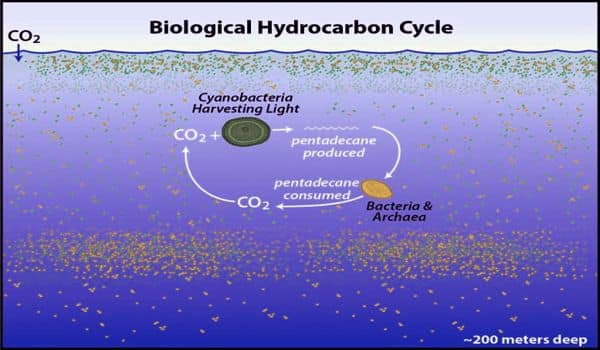
In 2015, an international team led by scientists at the University of Cambridge published a study showing that pentadecane hydrocarbon was formed by marine cyanobacteria in laboratory cultures. Researchers have extrapolated that this compound could be essential in the ocean. The molecule helps to reduce tension in curved membranes, so it is used in stuff like chloroplasts, where closely packed membranes require excessive curvature, Valentine explained. Some cyanobacteria also synthesize the compound, whereas other ocean microbes readily eat it for oil.
Valentine, along with Chris Reddy from Woods Hole, wrote a two-page commentary on the paper and wanted to explore the subject further with Arrington and Love. They explored the Gulf of Mexico in 2015, then the West Atlantic in 2017, collecting samples and conducting tests. The team sampled seawater from the nutrient-poor Atlantic area known as the Sargasso Sea, named for the floating sargasso algae from the Gulf of Mexico. This is gorgeous, pure, blue water with a hint of Bermuda in the center, Valentine said.
It was obviously a very tricky effort to obtain the samples. Since pentadecane is a natural hydrocarbon in diesel fuel, the crew had to take special measures to prevent pollution by the ship itself. They made the captain transform the ship into the wind so that the exhaust would not taint the samples and tested the chemical signature of the diesel to guarantee that it was not the cause of any pentadecane they detected.
Moreover, no one could drink, cook or paint on the deck whilst the researchers were gathering seawater. “It was a great deal,” Valentine said, “I don’t know if you’ve been on a ship for a long time, so you’ve been painting every day. It’s like the Golden Gate Bridge: you begin at one end, and when you get to the other end, it’s time to start again.”
The safeguards succeeded, and the team retrieved pristine samples of seawater. “After the 2017 expedition, standing in front of the gas chromatograph in Woods Hole, it was obvious that the samples were clean with no trace of diesel,” said co-leading author Love. “Pentadecane was unmistakable and showed simple oceanographic trends even in the first few samples that [we] ran.”
Because of their large numbers in the world’s ocean, Love added, “only two species of marine cyanobacteria contribute up to 500 times more hydrocarbons each year to the ocean than the amount of all other types of oil inputs into the ocean, including natural oil seps, oil spills, fuel dumps, and land runoff.” Collectively, these microbes generate 300-600 million metric tons of pentadecane per year, which is equivalent to 1,3 million metric tons of hydrocarbons emitted from all other sources.
While these quantities are remarkable, they are a little deceptive. The authors point out that the pentadecane cycle covers 40% or more of the Earth’s atmosphere, and more than one trillion quadrillion pentadecane-laden cyanobacterial cells are suspended in the sunlit portion of the world’s ocean. However, the life span of these cells is typically shorter than two days. As a result, researchers believe that the ocean holds only around 2 million metric tons of pentadecane at any given time. It’s a fast-spinning wheel, Valentine said, so the total volume present at any time is not particularly high. “Every two days you make and eat all the pentadecane in the ocean,” he said.
In the future, researchers expect to connect genomic microbes with their physiology and ecology. The team also has genome sequences for millions of animals that have multiplied to eat pentadecane in their samples. “The amount of knowledge there is amazing,” Valentine said, “and I think it shows just how much we don’t know about the ecology of a lot of hydrocarbon-consuming animals.”
Having accepted the nature and extent of this biohydrocarbon cycle, the team aimed to address the issue of whether its presence could lead the ocean to break down spilled petroleum. The main issue, Arrington explained, is whether these plentiful pentadecane-consuming microorganisms act as an asset during oil spill cleaning. To study this, pentane—petroleum hydrocarbon similar to pentadecane—has been applied to the seawater sampled at different distances from natural oil seams in the Gulf of Mexico.
They measured the total respiration of each sample to see how long pentane-eating microbes had taken to replicate. The researchers speculated that if the pentadecane cycle really used microbes to eat other hydrocarbons as well, then all the samples would produce blooms at comparable rates.
But that wasn’t the case. Samples from near-oil seeps soon produced flowers. “Although we added pentane for about a week, we’ve seen a large population growth,” Valentine said. “And that’s going to be slower and slower the more out you get, until, when you’re out in the North Atlantic, you can wait months and never see a flower.” In reality, after the expedition to Woods Hole, Massachusetts, Arrington had to remain behind in order to finish the experiment on samples from the Atlantic because those blooms had taken too long to emerge.
Interestingly, the team also found evidence that microbes belonging to another realm of life, Archaea, may also play a part in the pentadecane cycle. “We also discovered that a community of enigmatic, globally abundant microbes—which have yet to be domesticated in the laboratory—may be fuelled by pentadecane on the surface of the ocean,” said co-leader Arrington. The findings raise the question as to why the existence of an immense pentadecane cycle seemed to have little impact on the breakdown of the petrochemical pentane. “Oil is different from pentadecane,” Valentine said, “and you need to consider what the distinctions are, and what the compounds really make up oil, to understand how the microbes of the ocean can react to it.”
Ultimately, the genes widely used by microbes to eat pentane are isolated from those used by pentadecane. “A microbe living in clear waters off the coast of Bermuda is much less likely to encounter petrochemical pentane than pentadecane formed by cyanobacteria and is, therefore, less likely to bear pentane-consumption genes,” Arrington said.
Loads of different microbial species may consume pentadecane, but this does not mean that they may not consume other hydrocarbons, Valentine continued, particularly given the variety of hydrocarbon structures that occur in the petroleum sector. There are fewer than a dozen common hydrocarbons produced by marine animals, including pentadecane and methane. In the meantime, oil is made up of tens of thousands of various hydrocarbons. What is more, we are now finding that species capable of breaking down complex petroleum materials appear to exist in greater abundance along natural oil seams.
Valentine terms this process “biogeographic priming”—when the microbial population of the ocean is conditioned to a single source of energy in a given geographic region. “And what we see with this work is a difference between pentadecane and petroleum,” he said, “it is important to consider how different ocean regions can react to oil spills.”
Nutrient-poor gyres like the Sargasso Sea account for an incredible 40% of the Earth’s surface. But, ignoring the ground that already leaves 30% of the earth to be explored for other biohydrocarbon cycles. Valentine is of the opinion that systems in higher efficiency regions will be more dynamic and will perhaps provide more input for oil consumption. He also found out that the nature blueprint for the development of biological hydrocarbons is promising efforts to create the next wave of renewable energy.