Carriers of the Alzheimer’s disease-associated genetic mutation ADAD have less structural connectivity in the frontoparietal control network. According to a study published in Radiology, the structural integrity of the brain’s white matter as measured by an advanced MRI technique is lower in cognitively normal people who carry a genetic mutation linked to Alzheimer’s disease than in non-carriers. The findings, according to the researchers, demonstrate the promise of widely available imaging techniques in aiding in the understanding of early structural changes in the brain before symptoms of dementia become apparent.
People who have the autosomal dominant Alzheimer disease (ADAD) mutation are more likely to develop Alzheimer’s disease, a type of dementia that affects one out of every nine people in the United States. The mutation is linked to an abnormal protein called amyloid-beta buildup in the brain, which affects both gray matter and signal-carrying white matter.
“It’s thought that amyloid deposition in the gray matter could disrupt its function, causing the white matter to function incorrectly or even atrophy,” said study lead author Jeffrey W. Prescott, M.D., Ph.D., a neuroradiologist at MetroHealth Medical Center in Cleveland.
White matter integrity is destroyed in Alzheimer’s patients with a gene mutation. Carriers of the ADAD Alzheimer’s-associated genetic mutation have lower structural connectivity in the frontoparietal control network.
Dr. Prescott and colleagues discovered that white matter structural connectivity, as measured with an MRI technique called diffusion tensor imaging (DTI), degraded significantly as patients developed more amyloid burden in an earlier study on patients with sporadic Alzheimer’s disease, which accounts for 99 percent of cases.
“The current study extends these findings by demonstrating that similar findings are detectable in asymptomatic at-risk patients,” said Jeffrey R. Petrella, M.D., a Duke University professor of radiology and senior author on both studies.
Dr. Prescott and colleagues used data from the Dominantly Inherited Alzheimer Network (DIAN) to compare ADAD mutation carriers with non-carriers to see if there were changes in structural connectivity that could be related to the mutation.
The study included 30 mutation carriers, with an average age of 34 years, and 38 non-carriers, with an average age of 37 years. When structural brain MRI and DTI were performed on the participants, they all had normal cognition.
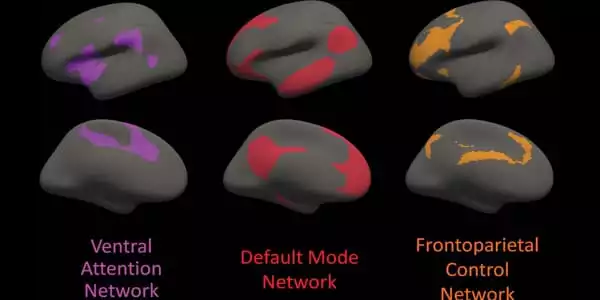
The frontoparietal control network, which connects areas primarily in the parietal and frontal lobes, two regions known to be involved in Alzheimer’s disease, was found to have lower structural connectivity in mutation carriers. Even after controlling for amyloid plaque burden, there was a correlation between expected years until the onset of symptoms and white matter structural connectivity in the frontoparietal control network among mutation carriers.
“This suggests that DTI network integrity measures may serve as a surrogate for the brain’s resilience to pathologic attack,” Dr. Petrella said. “We used a network measurement called global efficiency, and a decrease in efficiency can be interpreted as a breakdown in network organization,” Dr. Prescott added. “The findings show that as mutation carriers approach the estimated age of symptom onset, global efficiency decreases significantly.”
The study’s findings suggest that imaging-based identification of structural changes in the brain in people at genetic risk for early-onset Alzheimer’s disease could help researchers better understand how genes influence the disease process that leads to dementia.
“This demonstrates the potential of MRI as an evaluation tool in patients deemed at risk for Alzheimer’s disease before symptoms develop,” Dr. Prescott said. “The application of these advanced MRI techniques could help refine the identification of at-risk patients and risk measurements.”
The findings also suggest that imaging may play a role in the development of therapeutic drugs to treat Alzheimer’s disease. While most trials have been conducted on patients who already have Alzheimer’s disease or cognitive impairment, earlier identification and treatment of patients at risk represents a more promising avenue for preventing or at least delaying the onset of dementia.
“One potential clinical application of this study tool would be to add quantitative information to risk factors such as family history and use that to help identify patients early, when they may benefit from treatment,” Dr. Prescott explained. “However, we will have to wait for that to be implemented until we have an effective treatment.”
The researchers intend to conduct a follow-up study with advanced imaging and updated data from the DIAN network to assess the progression of Alzheimer’s disease in the study participants.