Suppose, a person is standing with a glass of water in his hand. Suddenly by dropping the glass on the floor, it was broken, as result water spilt on the floor. Now it is not possible at all to collect water and make the broken glass as before. In this case, working substance i.e., water cannot be recovered. Again the event has occurred very rapidly. It is an irreversible process.
So it can be said that the process which cannot be retracted in opposite direction by reversing is called irreversible process. It is also called indelible process. In this process, the system never shows the tendency to go back to its initial state. It is a very rapid process and thermodynamic equilibrium is not maintained.
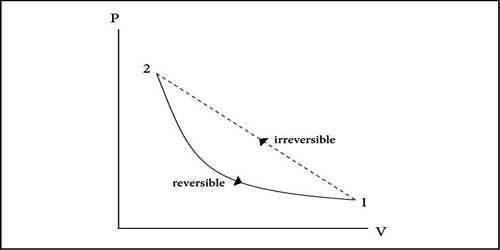
Or, The process in which applying all the available physical means, the whole system cannot be taken back to the initial state or, a process that cannot be made to go back in the reverse direction is called irreversible process. Death of a man may be considered as an irreversible process.
Some key points about the irreversible process:
- In the irreversible process, the original state of the system and environments cannot be renovated from the final state.
- During the irreversible process, the various states of the system on the path of change from preliminary state to ending state are not in equilibrium with each other.
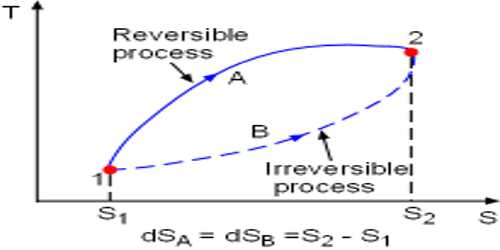
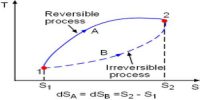
- During the irreversible process, the entropy of the system increases determinedly and it cannot be reduced back to its preliminary value.