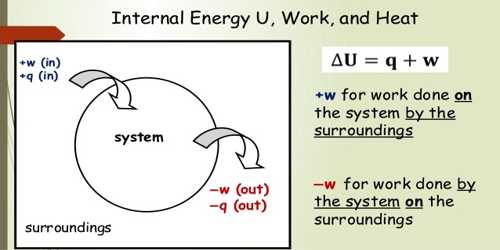
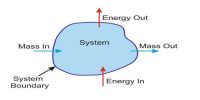
Internal Energy of a System
Put pressure by hand on a balloon filled with gas. You will see that the ballon is also giving pressure from inside on your hands. From where the ballon got this energy? This energy is the internal energy. From the above idea, we can say-
In each system, there is a fixed amount of energy which can be transformed into another energy to do work. Kinetic energy within the molecules and atoms of the body and the energy produced due to the intermolecular forces between them is combinedly called the internal energy.
In short, it can be said that the energy hidden within a system which can appear in different conditions is called the internal energy of the system.
Internal energy is the summation of the following two energies:
(a) Thermal energy that is the kinetic energy of the molecules which are in random motion and
(b) Atomic potential energy.
Atomic potential energy is originated from the interatomic force acting between the atoms of the molecule and intermolecular force between the molecules.
Total internal energy, E = Kinetic energy (K.E.) + Potential energy (P.E.).
Heat which flows from hot body to cold body is produced in the internal energy of the hot body. When heat flows from the hot body to told body due to the temperature difference, then the internal energy of the hot both decreases; on the other hand, the internal energy of the cold by increases. Actually, to designate the flow of energy from a hot body to a cold body the word heat is used. It is not correct to say that a body contains heat inside it. Actually, a body contains internal energy and not heat.