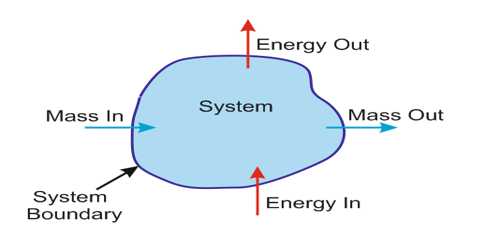
Thermodynamic system: A definite quantity of matter bounded by some closed surface is known as thermodynamic system whereat thermodynamic variables (pressure, volume, temperature) can be measured. For example, a cylinder having a piston or gas inside a ballon is called a system.
It is the substance and radiative content of a macroscopic volume in space, that can be sufficiently described by thermodynamic state variables such as temperature, entropy, internal energy, and pressure.
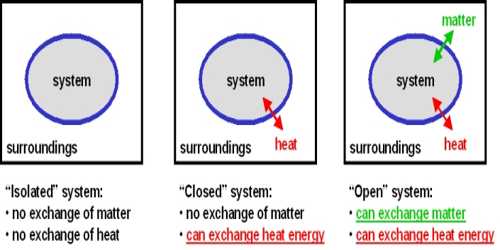
There are three major kinds of system: open system, closed system, and isolated system. All these have been described below:
Open system: The system in which the transfer of mass, as well as energy, can take place across its boundary is called as an open system.
Closed system: The system in which the transfer of energy takes place across its boundary with the surrounding, but no transfer of mass takes place is called a closed system. The closed system is the fixed mass system.
Isolated system: The system in which neither the transfer of mass nor that of energy takes place across its boundary with the surroundings is called an isolated system.