Thermodynamic process
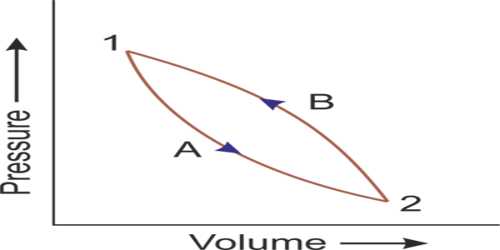
Any change of thermodynamic coordinates of a system is called the thermodynamic process. A thermodynamic process is a way of a thermodynamic system from an original to a final state of thermodynamic equilibrium.
Various kinds of thermodynamic processes are- isothermal procedure, adiabatic procedure, isochoric procedure, isobaric procedure, and reversible procedure. These have been described below:
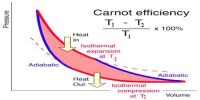
Isothermal procedure: When the system undergoes alter from one state to the other, but its temperature remains stable, the system is said to have undergone the isothermal process.
Adiabatic procedure: The procedure, during which the heat content of the system or definite quantity of the substance remains stable, is called an adiabatic procedure.
Isochoric procedure: The procedure, during which the volume of the system remains stable, is called an isochoric procedure. Heating of gas in a closed cylinder is an example of the isochoric procedure.
Isobaric procedure: The procedure during which the pressure of the system remains stable is called an isobaric procedure.
Reversible procedure: In simple words, the procedure which can be revered back totally is called a reversible procedure. This means that the ultimate properties of the system can be entirely reversed back to the original properties.