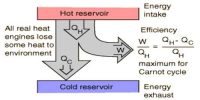
Principle of heat engine
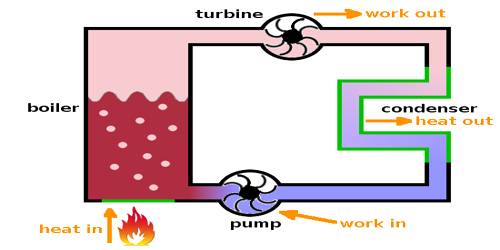
A heat engine is a device that converts heat to work. It takes heat from a reservoir then does some work like moving a piston, lifting weight, etc, and finally discharging some of the heat energy into the sink. In each engine, there is one working substance. For example, a vapor is a working substance in a vapor engine, again petrol is a working substance in a petrol engine. The working substance receives heat from a high-temperature source and a part of that heat is transformed into work and the rest amount of heat is respected in the heat sink at low temperature. This is the principle of the heat engine.
That means the engine by which heat energy can be transformed into mechanical energy is called a heat engine. For example, vapor engine, petrol engine, diesel engine, etc. In general, a heat engine is a device that converts chemical energy into heat or thermal energy and then to mechanical energy or to electrical energy.
The temperature of the source from where heat is received by the engine should be higher than the temperature of the heat sink. That means, the engine receives heat from a higher temperature source, a part of that heat is transformed into work and the rest amount is rejected to the heat sink at a lower temperature and the engine comes back to the initial state. To get work continuously from the engine the cycle is to be changed in this way.
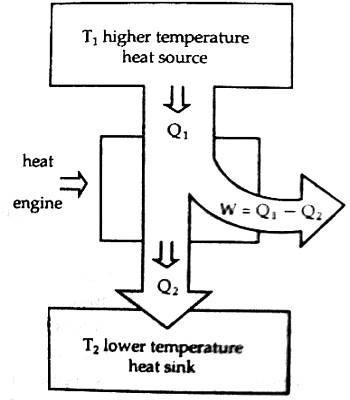
According to figure, the working substance receives Q amount of heat from the source at temperature T. This engine does work by a portion of heat in energy into mechanical energy and some position of heat is rejected into the heat sink and becomes cold so that the engine can receive heat again from the source. If the amount of heat rejected in the heat sink is Q2 at temperature T2, the amount of heat used for transforming into work is, W = Q1 – Q2. The engine which can convert a larger portion of heat energy into work has higher efficiency. A petrol engine has higher efficiency than a vapor engine.
Uses
This device typically uses the energy provided in the form of heat to do work and then exhausts the heat which cannot be used to do work. Thermodynamics is the study of the relationships between heat and work. The first law and second law of thermodynamics constrain the operation of a heat engine.
Types
- External combustion engine – In these heat engines, the fuel burns outside and away from the main engine where force and motion are produced. A steam engine is an example of an external combustion engine.
- Internal combustion engine – In these heat engines, the fuel burns inside the cylinder. A car engine is an example of an internal combustion engine.
Internal combustion engines are generally far more efficient than external combustion engines because no energy is wasted transmitting heat from a fire and boiler to the cylinder; everything happens in one place.