Conditions and Characteristics of isothermal changes
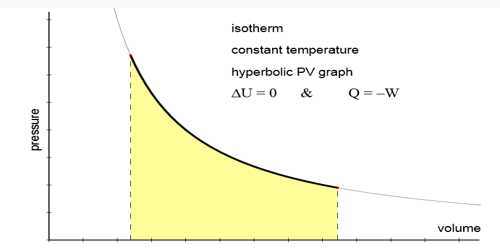
The change in which pressure and volume of a gas are changed but the temperature remains constant that change is called isothermal change and the process by which this change occurs is called isothermal process.
Conditions for isothermal changes
- The gas is to be kept in a good conducting vessel or container.
- Heat capacity of the media surrounding the container must be very high,
- Change of pressure is to be made slowly and slowly.
- Temperature is to be maintained constant either by supplying or by removing
Characteristics of isothermal change
- Change of pressure and volume of a gas by keeping the temperature constant is called isothermal change.
- In this change, necessary heat is either supplied or removed.
- It is a very slow process.
- In this change, the container should have good thermal conductivity.
- All surroundings of the container should have a very high thermal capacity.
- Isothermal change obeys Boyle’s law i.e., PV = constant.
- The isothermal curve is comparatively less steep.