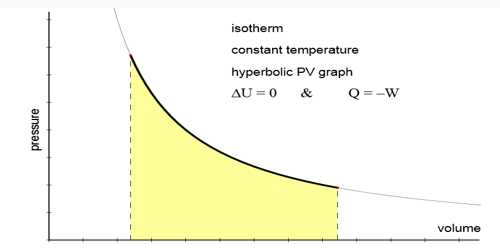
Isothermal change
This is a change where the temperature of the gas remains constant and to achieve this we will have to either add or remove heat from the gas during the change – adding heat energy if is expanded and removing if it is compressed. It is an experimental fact that when a gas is compressed suddenly by applying pressure a certain amount of heat is produced. As a result temperature of the gas increases. But if the gas is very slowly compressed and produced heat is removed instantaneously then there will be no change of temperature i.e., the temperature of the gas will remain constant.
Again if a gas is expanded suddenly it loses some amount of heat during doing work against the external pressure. Consequently, its temperature will decrease. But if the gas is expanded very slowly and necessary heat is supplied from outside, then the temperature will remain constant. This type of change is called isothermal change. So it is seen that during isothermal change the temperature is kept constant either by supplying heat to a gas or sometimes by removing heat from the gas.
That means the change in which pressure and volume of a gas are changed but the temperature remains constant that change is called isothermal change and the process by which this change occurs is called isothermal process.
In isothermal process pressure and volume of the gas obey Boyle’s law. That means, P ∞ 1/V or PV = constant, where P and V are respectively pressure and volume.
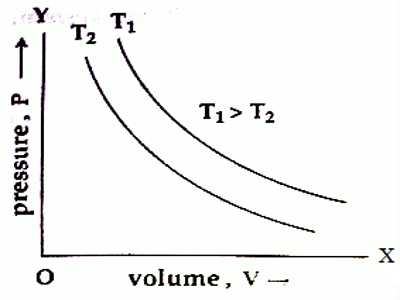
If a graph is drawn at the constant temperature, T by plotting volume of an ideal gas along X-axis and pressure along Y-axis, then the graph will be a rectangular hyperbola [Figure]. For different temperatures, different curves of same types are found. These graphs are called isothermal graphs.