Measurement of Lowering of Vapour pressure
A number of methods for the determination of vapour pressure of liquids are not suitable for accurate measurement of the lowering of vapour due to addition of solute, because the change in vapour pressure is rather small. In order to avoid this difficulty vapour pressures may be measured at elevated temperatures where both p° and p are high. But even then sufficient precision is not assured. However, a new type of manometer, known as differential manometer, has been designed F to directly measure (p0 – p). A high precision differential manometer has been designed by Rayleigh and used by Frazer and Lovelace for measuring small differences in vapour pressure of the solvent and solution.
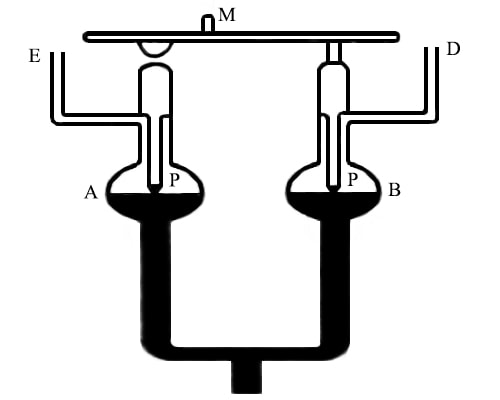
Figure: Differential manometer designed by Rayleigh
The manometer (figure) essentially consists of small glass bulbs, A and B, which are connected by a movable mercury reservoir. Two glass points PP, are sealed to the bulbs which are securely fixed to the horizontal beam carrying a minor, M. The bulbs are connected to side tubes, D and E. pressure in the two bulbs are made equal by mean of fine screws (not shown) by adjusting the height of the mercury. This is achieved when the two glass pins exactly touch the mercury surface. This can be ascertained by observing the image of the glass pins on the clean mirror-like mercury surface. This corresponds to zero reading of the manometer. One of the two bulbs is then connected to the vapour of the solution and the other to that of the solvent through the side tubes D and E. Due to the difference in the vapour pressure, the mercury levels in two bulbs are displaced. The level of mercury is brought back to the initial positions by carefully tilting the apparatus and adjusting the reservoir till PP again coincides with their images. When the instrument has been tilted the mirror M has also been tilted. The magnitude of this tilt is measured by the displacement of the reflected light from the mirror, a method known commonly as ‘lamp and scale arrangement”. The extent of the tilt is a measure of the vapour pressure difference between the solution and the solvent. By careful work, it is claimed to give vapour pressure difference with an accuracy of about 0.0006 mm of mercury.