The retroviral lifestyle necessitates the virus finding suitable host cells to establish persistent infection and evading the host’s immune response. Understanding each stage of a virus’s life cycle is critical for identifying potential treatment targets. Scientists have now demonstrated how a virus from the retrovirus family, which includes HIV, protects its genetic information and becomes infectious. Furthermore, they demonstrate the virus’s unexpected flexibility.
Retroviral infection is typically lifelong, owing to retroviral genomes’ proclivity to irreversibly integrate into host DNA. Viruses are the ultimate molecular machines. Their only goal is to insert their genetic material into healthy cells and multiply as a result. They can thus cause diseases that kill millions of people and keep the world on edge with deadly precision. HIV, which is the cause of the ongoing global AIDS epidemic, is one example of such a virus, though it is currently less discussed. Despite recent progress, 690,000 people died as a result of virus infection in 2019 alone.
Retroviruses are a type of virus that is classified based on how it is structured and how it replicates within a host. Aside from the virus that causes AIDS, human immunodeficiency virus (HIV), there are two other retroviruses that can cause human illness. One is known as human T-lymphotropic virus type 1 (HTLV-1) and the other as human T-lymphotropic virus type 2 (HTLV-2) (HTLV-II). Both of these viruses are spread between people through sexual contact, infected blood or tissue exposure, or from an infected person to their child during pregnancy or childbirth.
Scientists are able to show how a virus from the retrovirus family – the same family as HIV – protects its genetic information and becomes infectious.
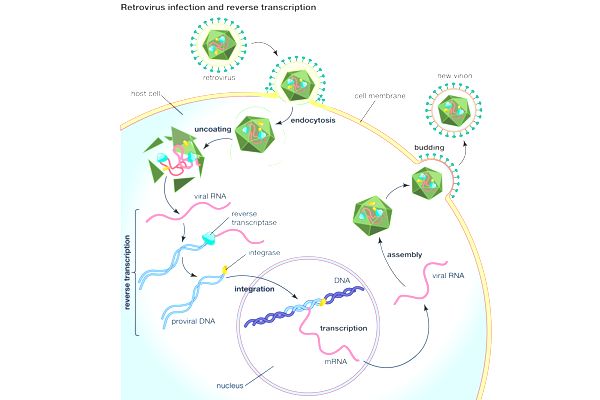
Reverse transcriptase converts the retroviral RNA genome into double-stranded DNA after it enters a host cell. This viral DNA then migrates to the nucleus and integrates with the host genome. The genes of viruses are transcribed and translated. New virus particles assemble, exit the cell, and have the potential to infect another cell.
“If you want to know the enemy, you have to know all of its friends,” says Martin Obr, a postdoc at IST Austria’s Schur group. As a result, he and his colleagues are researching a virus related to HIV, the Rous sarcoma virus, which causes cancer in poultry. With its assistance, he was able to gain new insights into the critical role that a small molecule plays in the assembly of these viruses.
Protecting the virus blueprint
The team, along with collaborators from Cornell University and the University of Missouri, focused on the late phase of retrovirus replication in their study, which was published in the journal Nature Communications. “It takes a long time from an infected cell to a mature virus particle that can infect another cell,” first author Martin Obr explains.
In an immature, non-infectious state, a new particle buds from the cell. It then forms a protective shell around its genetic information, known as a capsid, and becomes infectious. This protective shell is made up of hexamers and a few pentamers of a protein. The researchers discovered that a small molecule known as IP6 plays an important role in the stabilization of the protein shell within the Rous sarcoma virus.
“If the protective shell is not stable, the virus’s genetic information may be released prematurely and destroyed, but if it is too stable, the genome cannot exit at all and thus becomes useless,” explains Assistant Professor Florian Schur. In a previous study, he and his colleagues demonstrated that IP6 plays an important role in the assembly of HIV.
Now, the researchers have demonstrated that it is just as important in other retroviruses, demonstrating how important the small molecule is in the virus life cycle. “When you build a car, you have all these big metal parts, like the hood, roof, and doors — the screws connect everything.” In our case, the big parts are the capsid proteins and the IP6 molecules are the screws,” says Obr.
Unexpected flexibility
The team was able to see how variable the shapes formed by capsid proteins are by further developing cryo-electron tomography, a technique that allows scientists to look at extremely small samples in their natural state. “Now we wonder why the virus changes the shape of its capsid. To what is it adapting? “Martin Obr, a postdoctoral researcher, agrees. Different capsid shapes within the same virus type could indicate differences in virus particle infectivity. “Everything happens for a reason,” says Florian Schur, “but there is no clear answer yet.” Further developing the technology to get to the bottom of these highly optimized pathogens remains a challenging and fascinating task for the scientists.