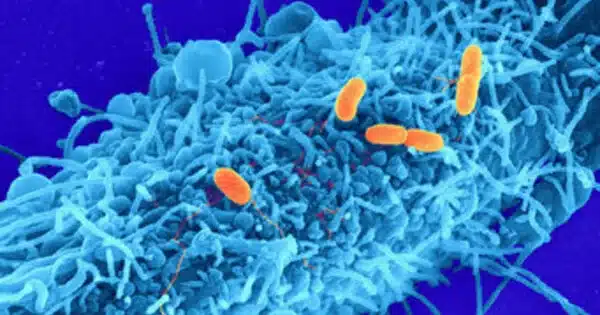
You suddenly feel sick because dangerous germs have colonized and proliferated throughout your body! They deploy toxic poisons as their invading weapons, targeting the host’s defense mechanisms and key cell activities. Before these lethal toxins can target host cells, bacteria must export them from their producing site, the cytoplasm, via specific secretion mechanisms.
Stefan Raunser, Director of the Max Planck Institute of Molecular Physiology, and his team have discovered a previously unknown, remarkable secretion mechanism that allows the release of massive Tc poisons. In a kamikaze strike, a tiny group of so-called “soldier” germs, packed to the brim with toxins, explode in the host, releasing their lethal cargo. Targeting such subpopulations in medical therapies could be an effective therapy strategy for diseases caused by bacteria that are becoming increasingly resistant to antibiotics.
When a pathogenic bacterium enters its host, it activates a succession of defensive and attack processes to disseminate, penetrate, and colonize deeper tissues and organs. This involves the production of a variety of harmful proteins that disrupt the host’s cellular defenses. Toxic proteins in gram-negative bacteria, which can cause serious infections and are growing increasingly resistant to antibiotics, must pass many cellular barriers — both bacterial and host — before reaching their goal. Bacteria have evolved a variety of specific secretion systems to do this.
We believe that normal cells transform into soldier cells upon ingestion in response to insect host nutrition. Toxin secretion is pH sensitive, thus it is not released until the soldier cells reach the alkaline posterior midgut, their primary theatre of action.
Stefan Raunser
Some can secrete a variety of toxins and are found in almost all bacteria, while others have been identified in only few bacteria. The machinery for the secretion of many smaller toxins has already been established. Not so for larger ones, like the Tc toxins produced by the notorious Yersinia bacteria, which also include pathogens that cause plague and tuberculosis.
“It has remained enigmatic for decades how the huge Tc toxins reach their final destination. By obtaining the first 3D structures of a Tc toxin in our previous electron cryomicroscopy studies we could already figure out how it bypasses the last barrier, the host membrane, using a syringe-like injection mechanism. Now, we were able to complete the picture and show how these toxins overcome the three barriers separating the inside of the bacteria from its environment in a truly spectacular way,” says Stefan Raunser.
Exploding bacteria
In their current work, Raunser and his team used a cutting-edge mix of multiple approaches to investigate the production of the Tc toxin YenTc generated by the insect pathogen Yersinia entomophaga, which is critical for this bacterium species’ infection. The most difficult task was originally determining which of the known secretion machinery is used for this purpose by the bacterium.
To do this, the scientists used targeted genome editing to eliminate all putative secretion systems one by one. When none of the knockouts prevented the toxin from being released, the same procedure was employed to tweak the toxin so that its secretion could be visualized — this time successfully.
“Watching some of the bacteria literally explode to release their toxins was a real eureka moment,” says Oleg Sitsel, first author of the study. Careful proteomic analysis then finally brought to light a pH-sensitive type 10 secretion system responsible for toxin release, a class of protein export machinery that was just recently established. Subsequent cryo-electron tomographic analysis visualized the step-by-step details of how this secretion system exports cellular contents via a previously unknown lytic mode of action that overcomes the three barriers surrounding gram-negative bacteria.
Becoming a soldier cell
The scientists found that only a small specialized subset of bacterial cells produces and exports the toxins by paying the ultimate price, namely death. But what causes those cells, which the authors termed “soldier cells,” to first enlarge and produce a deadly toxin cocktail containing YenTc, then commit suicide for the benefit of their comrades?
The researchers first discovered that the appearance of soldier cells is temperature, nutrition, and cell density-dependent. They subsequently uncovered a temperature-sensitive genetic switch that coordinates the production of poisons with the secretion system, transforming “normal” cells into soldier cells. The enormous production of toxins, along with the cells’ larger size, means that only a few individuals must be lost for the greater welfare of the bacterial community, making this an incredibly efficient technique.
“We believe that normal cells transform into soldier cells upon ingestion in response to insect host nutrition. Toxin secretion is pH sensitive, thus it is not released until the soldier cells reach the alkaline posterior midgut, their primary theatre of action,” Raunser explains.
“This secretion technique is novel and amazing. These bacteria’s behaviour resembles that of eusocial systems, including differentiation and altruism. If this is a more common mechanism, we may have discovered a weakness in bacteria: specifically targeting soldier cells could become a promising medical strategy in the fight against pathogenic bacteria, especially in an era of increasing antibiotic resistance,” concludes Raunser.