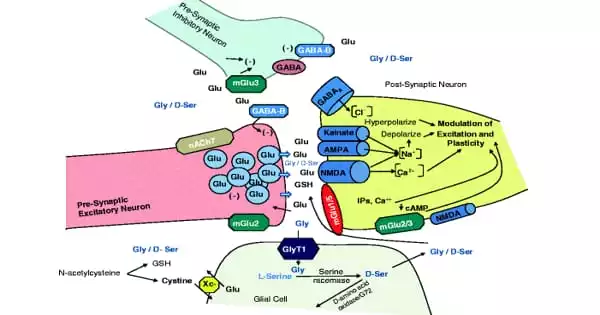
Glycine has the ability to stimulate or inhibit neurons in the brain, allowing it to control complex functions. Researchers have taken a significant step toward understanding the regulation of glycine in the brain by unraveling the three-dimensional structure of the glycine transporter. These findings suggest that effective drugs that inhibit GlyT1 function could be developed, with significant implications for the treatment of schizophrenia and other mental disorders.
Glycine is the smallest amino acid and a protein building block, as well as a critical neurotransmitter that can both stimulate and inhibit neurons in the brain, controlling complex brain functions. Glycine transporters, which reuptake and clear glycine from synapses between neurons, mediate the termination of a glycine signal. Transporter of glycine GlyT1 is the primary regulator of neurotransmitter glycine levels in the brain, as well as in blood cells, where glycine is required for heme synthesis.
Glycine activates the N-methyl-D-aspartate (NMDA) receptor, and its poor performance has been linked to schizophrenia. Many pharmaceutical companies and academic research laboratories have thus focused on influencing glycinergic signaling and glycine reuptake delay as a means of activating the NMDA receptor in search of a cure for schizophrenia and other psychiatric disorders over the last two decades.
Glycine transporters, which reuptake and clear glycine from synapses between neurons, mediate the termination of a glycine signal. Transporter of glycine GlyT1 is the primary regulator of neurotransmitter glycine levels in the brain, as well as in blood cells, where glycine is required for heme synthesis.
Azadeh Shahsavar
Indeed, several potent and selective GlyT1 inhibitors have antipsychotic and pro-cognitive effects that alleviate many symptoms of schizophrenia and have progressed into clinical trials. However, a viable drug candidate has yet to emerge, and GlyT1 inhibition in blood cells raises concerns about potential side effects. Insight into the structure of inhibitor binding to GlyT1 would aid in the discovery of new drug design strategies.
To better understand the three-dimensional structure and inhibition mechanisms of the GlyT1 transporter, researchers from Roche and Linkster, as well as the European Molecular Biology Laboratory (EMBL) Hamburg, University of Zurich, and Aarhus University, have collaborated on the investigation of one of the most advanced GlyT1 inhibitors. The researchers were able to grow microcrystals of the inhibited GlyT1 complex using a synthetic single-domain antibody for GlyT1.
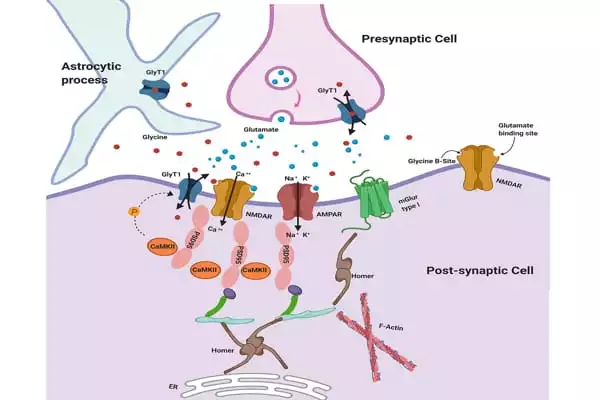
Using X-ray diffraction data from hundreds of microcrystals, the team led by Assistant Professor Azadeh Shahsavar and Professor Poul Nissen from the Department of Molecular Biology and Genetics/DANDRITE, Aarhus University, determined the structure of human GlyT1 using a Serial Synchrotron Crystallography (SSX) approach. The SSX method is particularly well-suited as a method for developing new, powerful X-ray sources, and it opens up new avenues for, among other things, drug development for a variety of applications.
The researchers tested 960 different conditions and were successful in obtaining GlyT1 crystals in one of them. “The crystals were extremely small and difficult to visualize.” “We chose to measure them at EMBL Hamburg’s beamline P14 because it is well suited for difficult experiments like this one,” Azadeh says. The X-ray beam at P14 is particularly strong and focused, and its equipment is designed to work with crystals as small as micrometers. However, the quality of the crystals varied, making data collection difficult. Azadeh’s perseverance eventually paid off. “I recall the first time I saw the inhibitor’s electron density. I was so excited, I couldn’t sleep for two nights,” she says. “You live for those rewarding moments.”
The structure was published in the prestigious scientific journal Nature, and it reveals a new inhibition mechanism in neurotransmitter transporters in general. Mechanisms for inhibiting the serotonin transporter (which has many similarities to GlyT1) with antidepressant drugs have previously been discovered, but a completely different inhibition mechanism for GlyT1 has now been discovered.
It provides a foundation for the future development of small molecules and antibodies as selective inhibitors of GlyT1, as well as new ideas for the development of inhibitors of other neurotransmitter carriers that can be used to treat other mental disorders. Azadeh Shahsavar’s research team will continue to look into GlyT1’s function and inhibition, as well as the effects of GlyT1 inhibitors in the body.
GlyT1 proved particularly difficult to study because it is unstable when extracted from the cell membrane. Scientists combined several approaches to stabilize it, such as creating more stable variants of the protein. To capture the transporter in a clinically relevant state, the researchers used a Roche-created chemical that binds and stabilizes GlyT1 from the inside, as well as a synthetic mini-antibody (sybody) that binds it from the outside.