Using cutting-edge imaging techniques on in vitro viral cells, researchers found a new role of anti-HIV-1 antibodies. The researchers discovered that some antibodies that have previously been shown to efficiently target the HIV-1 envelope (Env) protein can inhibit infected cells from releasing viral particles, hence preventing viral propagation. The findings show that, in addition to neutralization, these potent antibodies have a variety of antiviral properties.
Teams from the Institut Pasteur, CNRS, Vaccine Research Institute (VRI), and Université de Paris have uncovered a novel function of anti-HIV-1 antibodies by employing cutting-edge imaging techniques to in vitro viral cells. This chain of antibodies and viral particles works to keep viruses from spreading. These data show that, in addition to neutralization, these potent antibodies have other antiviral properties. The findings were reported in the journal Nature Communications.
Broadly neutralizing antibodies (bNAbs) that target the virus envelope (Env) protein show great therapeutic potential for HIV-1. They were first discovered in a small number of patients whose serum was capable of blocking multiple HIV strains. These antibodies have a wide range of antiviral properties. As well as neutralizing the virus, i.e. preventing it from infecting new cells, they also kill infected cells. As a result, they are known as polyfunctional molecules. To use current antibodies more effectively or to refine the selection criteria for new antibodies, it is vital to thoroughly understand the spectrum of their antiviral actions. Furthermore, greater research into the polyfunctionality of anti-HIV-1 antibodies is beneficial in order to expand our understanding of the role of antibodies and thereby combat different viral diseases.
We found that only the most potent antibodies bind to virus particles on the surface of infected cells. Trapped virus particles are no longer capable of infecting new cells. This study discovered a novel antiviral activity capable of broadly neutralizing anti-HIV-1 antibodies.
Olivier Schwartz
Initially, researchers from the Institut Pasteur, CNRS, VRI, and Université de Paris wanted to see if antibodies could block infected cells from creating viral particles. To that end, scientists grew CD4 T cells (HIV’s natural target) in vitro for 24 hours using several antibodies. They then examined the amount of viral particles produced by the cells in the culture media as well as the amount of viral particles remaining in the cells. The scientists were able to demonstrate that certain antibodies increased virus quantity in cells but decreased it in the culture medium as a result of these experiments. This surprising discovery led them to conclude that specific antibodies hampered viral particle release without stopping viral particle synthesis.
To put this notion to the test, the researchers observed the generation of virus particles by cells using several microscopy techniques. They first analyzed the cells using fluorescence microscopy, a technique used to distinguish viral proteins. This allowed them to show that infected cells collect a considerable amount of mature viral protein. This discovery implies that complete virus particles accumulate in cells.
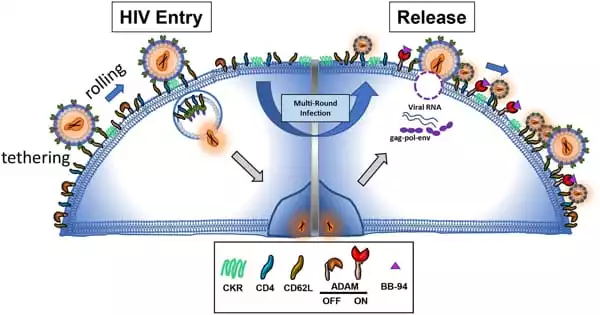
The scientists next utilized scanning electron microscopy to examine the surface of infected cells to pinpoint the specific position of these viral particles. “We found that these antibodies (bNAbs) cause an accumulation of viral particles at the surface of cells, generating clusters and very unusual structures (see picture),” says Timothée Bruel, co-last author of the paper and scientist at the Institut Pasteur’s Virus and Immunity Unit.
The researchers then combined transmission electron microscopy with immunogold labeling. This allowed them to show that antibodies create a chain cluster by interposing themselves between virus particles and the infected cell. Experiments with mutant antibodies later revealed that the antibodies’ Y shape is responsible for the clustered structure. Their arms can connect two viruses or one virus to the infected cell membrane, and their attachment points are strong enough to cause this event.
“We found that only the most potent antibodies bind to virus particles on the surface of infected cells. Trapped virus particles are no longer capable of infecting new cells “The study’s co-last author and Head of the Virus and Immunity Unit at the Institut Pasteur, Olivier Schwartz, concludes.
This study discovered a novel antiviral activity capable of broadly neutralizing anti-HIV-1 antibodies. It adds to our understanding of the mechanisms of action of these antibodies and explains their efficacy in clinical studies. The researchers are now looking at antibodies that target other viruses, including SARS-CoV-2, to see if they may also prevent viral spread through this mechanism.