T-cell immunotherapy has been a fascinating and fast-expanding field in the treatment of cancer and other immunological disorders. New techniques to nongenetic T-cell immunotherapy have emerged, and other breakthroughs may have occurred since then.
Cancer immunotherapies try to stimulate the immune system to fight cancer cells more effectively. A Chinese research team has recently presented a new, modular technique for T-cell-based immunotherapy that works without significant genetic changes in the journal Angewandte Chemie. Modulation of cell-cell communication via a clever regulatory circuit employing a variety of tiny, specifically folded DNA molecules (aptamers) permits cancer cells to directly activate their lethal adversaries, T cells.
Multicellular organisms, such as our bodies, require cells to “coordinate” with one another in order to function properly. Signals are sent and received, processed, and passed on in this intricate communications network. This process is aided by the regulation of certain membrane receptors that bind to signal molecules.
Multicellular organisms, such as our bodies, require cells to “coordinate” with one another in order to function properly. The goal is to create a “short circuit” in the communication channels, allowing tumor cells to activate T cells directly without passing via APCs.
Weihong Tan and Liping Qiu
In a typical case, immune system components known as antigen-presenting cells (APCs) detect the presence of cancer antigens. They send the signal to lymph nodes, where certain T lymphocytes are activated by their receptors, enter the circulation, and attack cancer cells. Cancer cells, unfortunately, exploit a variety of “loopholes” to avoid detection by the immune system.
Hunan University, Hangzhou Institute of Medicine, the Chinese Academy of Sciences, and Shanghai Jiao Tong University are collaborating to close these gaps. Their goal is to create new cellular interactions without using genetically engineered immune cells or receptors. The goal is to create a “short circuit” in the communication channels, allowing tumor cells to activate T cells directly without passing via APCs.
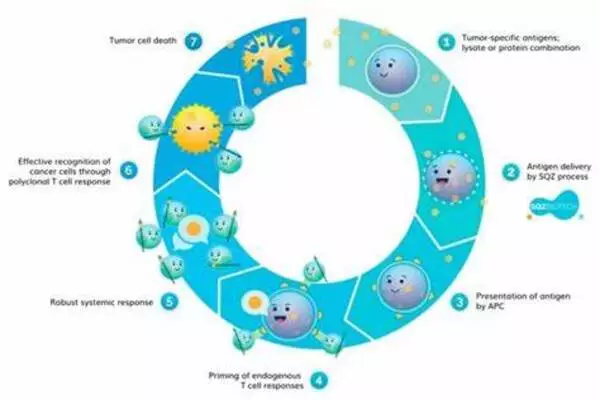
The team, led by Weihong Tan and Liping Qiu, created a “regulatory circuit” with two modules: 1) “recognition-then-triggering” and 2) “aggregation-then-activation.” The circuit is built around various DNA aptamers, which are short DNA segments that fold into a “preprogrammed” 3D form and “recognize” specific target molecules.
Module 1’s DNA is initially inactive and partially linked into a double strand. If cancer cells are present, the aptamer part of the “recognition” single strand binds to protein tyrosinase kinase 7, a protein found on the surface of many cancer cells in huge quantities. This causes the double strand of DNA to separate, releasing the “triggering strand,” which activates module 2).
Module 2 necessitates the use of two additional aptamers. Both bind to the CD28 immunoreceptors on the surfaces of T lymphocytes. CD28 is a co-stimulator of T cell activation. This activating strand binds to an extra “loop” on a type 1 aptamer. The loop closes, and the freshly released end attaches to a type 2 aptamer, which binds to another type 1 aptamer, and so on (hybridization). This causes a double strand to form, and the attached CD28 receptors to consolidate, generating a signal cascade that greatly enhances T cell activation. As a result of “short-circuited” cell communication, cancer cells can extremely effectively lead T lymphocytes to attack them.