Living cell factories, where biological enzymes do the work, can produce drug molecules and biofuels on demand. Chalmers University of Technology researchers have developed a computer model that can predict how fast enzymes work, allowing them to find the most efficient living factories and study difficult diseases.
Enzymes are proteins that can be found in all living cells. Their role is to act as catalysts, increasing the rate of specific chemical reactions that occur in cells. Enzymes, which can be compared to nature’s small factories, thus play an important role in making life on Earth work. They are also used in detergents, as well as the production of sweeteners, dyes, and medicines. The potential uses are almost endless, but are hindered by the fact that it is expensive and time-consuming to study the enzymes.
“To study every natural enzyme with experiments in a laboratory would be impossible, they are simply too many. But with our algorithm, we can predict which enzymes are most promising just by looking at the sequence of amino acids they are made up of,” says Eduard Kerkhoven, researcher in systems biology at Chalmers University of Technology and the study’s lead author.
To study every natural enzyme with experiments in a laboratory would be impossible, they are simply too many. But with our algorithm, we can predict which enzymes are most promising just by looking at the sequence of amino acids they are made up of.
Eduard Kerkhoven
Only the most promising enzymes need to be tested
The enzyme turnover number, also known as the kcat value, describes how quickly and efficiently an enzyme works and is critical for understanding cellular metabolism. Chalmers researchers created a computer model that can quickly calculate the kcat value in their new study. The only information required is the order of the amino acids that make up the enzyme, which is frequently available in open databases. Following the model’s initial selection, only the most promising enzymes must be tested in the lab.
Given the abundance of naturally occurring enzymes, the researchers believe the new calculation model will be extremely useful.
“We see many possible biotechnological applications. As an example, biofuels can be produced when enzymes break down biomass in a sustainable manufacturing process. The algorithm can also be used to study diseases in the metabolism, where mutations can lead to defects in how enzymes in the human body work,” says Eduard Kerkhoven.
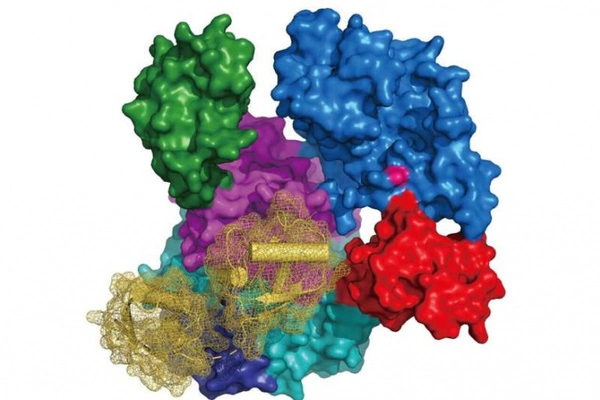
More knowledge on enzyme production
More possible applications are more efficient production of products made from natural organisms, as opposed to industrial processes. Penicillin extracted from a mould is one such example, as well as the cancer drug taxol from yew and the sweetener stevia. They are typically produced in low amounts by natural organisms.
“The development and manufacture of new natural products can be greatly helped by knowledge of which enzymes can be used,” says Eduard Kerkhoven.
The calculation model can also point out the changes in kcat value that occur if enzymes mutate, and identify unwanted amino acids that can have a major impact on an enzyme’s efficiency. The model can also predict whether the enzymes produce more than one “product.”
“We can find out if the enzymes have any’moonlighting’ activities and produce undesirable metabolites. It is useful in industries where a single pure product is frequently desired.”
The researchers put their model to the test by simulating metabolism in over 300 different yeasts using 3 million kcat values. They developed computer models to predict how quickly yeasts could grow or produce specific products, such as ethanol. The researchers concluded that models with predicted kcat values could accurately simulate metabolism when compared to measured, prior knowledge.