Triatomic hydrogen, abbreviated H3, is an unstable triatomic molecule composed entirely of hydrogen. It is a triatomic molecule, which means it contains three atoms. Because it contains only three hydrogen atoms, it is the simplest triatomic molecule, and the quantum mechanics description of the particles is relatively simple to numerically solve. Because the molecule is unstable, it disintegrates in less than a millionth of a second. It is rare due to its short lifetime, but it is formed and destroyed quite frequently in the universe due to the abundance of the trihydrogen cation.
Because of vibration and rotation, the infrared spectrum of H3 is very similar to that of the ion, H+3. This ability to emit infrared light allowed primordial hydrogen and helium gas to cool and form stars in the early universe.
In its stable state, hydrogen typically forms diatomic molecules (H2), which consist of two hydrogen atoms bonded together via a covalent bond. Each hydrogen atom in this configuration shares an electron with the other, resulting in a stable molecule. The bonding of three hydrogen atoms in triatomic hydrogen (H3) would necessitate new types of chemical bonds or interactions to form and stabilize the molecule.
Properties
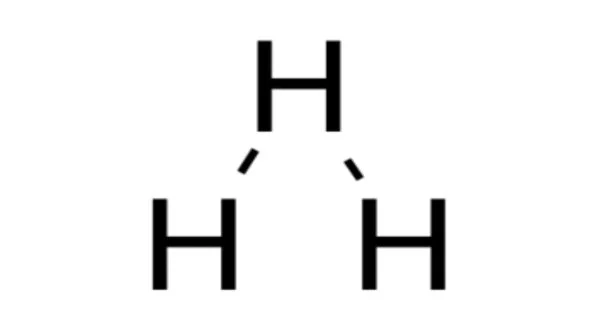
- Structure: An equilateral triangle with three hydrogen atoms evenly spaced apart is the most stable theoretical structure for triatomic hydrogen.
- Bonding: In the triatomic hydrogen molecule, each hydrogen atom forms a covalent bond with the other two hydrogen atoms, resulting in a three-center, two-electron bond. The bonding mechanism involves electron sharing among all three atoms, resulting in a delocalized electron cloud.
- Stability: H3 is thought to be less stable than diatomic hydrogen (H2). The presence of triatomic hydrogen is expected to be brief, and it is thought to dissociate quickly into diatomic hydrogen molecules.
- Energy: Triatomic hydrogen is predicted to have higher energy levels than diatomic hydrogen due to the increased number of interactions between the hydrogen atoms. The extra electron sharing and repulsion between atoms contribute to the higher energy state.
- Spectroscopic properties:Theoretical calculations suggest that triatomic hydrogen may exhibit characteristic vibrational and rotational energy levels. These energy levels can be probed using spectroscopic techniques, which could potentially provide insights into the molecule’s properties if it were to be observed experimentally.
Reactivity
Triatomic hydrogen is expected to have different chemical properties than diatomic hydrogen. Its increased complexity and electron distribution may influence its interactions with other molecules, resulting in different reaction pathways and rates.
The existence of triatomic hydrogen has been proposed in theory under certain extreme conditions, such as high-pressure environments or extremely low temperatures. These conditions may permit the formation of H3 via novel molecular configurations and bonding arrangements. However, experimental evidence for triatomic hydrogen’s existence is still lacking.