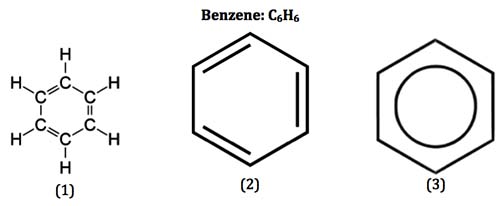
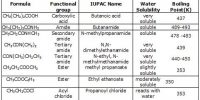
The aromatic compounds apparently contain alternate double and single bonds in a cyclic structure, and resemble benzene in chemical behaviour. They undergo substitution rather than addition reactions. This characteristic behaviour is called aromaticity.
Aromaticity is in fact a property of the sp2 hybridized planar rings in which the p orbitals (one on each atom) allow cyclic delocalization of π electrons.
Criteria for aromaticity :
On the basis of the above considerations, it can be laid down criteria or rules which help us in knowing whether a particular compound is aromatic or non-aromatic.
- An aromatic compound is cyclic and planar.
- Each atom in an aromatic ring has a p orbital. These p orbitals must be parallel so that a continuous overlap is possible around the ring.
- The cyclic π molecular orbital (electron cloud) formed by overlap of p orbitals must contain (4n + 2) π electrons, where n, integer = 0. 1. 2. 3 etc. This is known as Huckel rule.