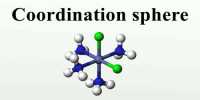
The coordination number of a metal ion in a complex can be defined as the number of ligand donor atoms to which the metal is directly bonded. Numerically coordination number represents the total number of the chemical bonds formed between the central metal ion and the donor atoms of the ligands.
For example, in K4 [Fe(CN)6] the coordination number of Fe(II) is 6 and in [Cu(NH3)4]SO4 the coordination number of Cu(II) is 4.