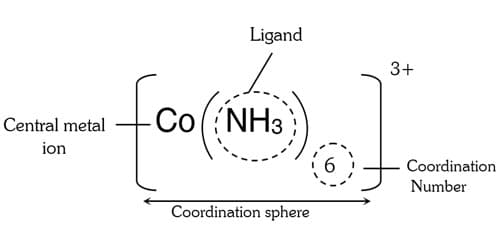
Central Metal Ion: In the complex ion an acceptor accepts a pair of electrons from the donor atoms. The acceptor is usually a metal/metal ion to which one (or) more of neutral molecules (or) anions are attached. The acceptor metal cation is referred to as central metal cation. Hence, central metal cation in a complex serves as a lewis acid.
The central metal ion is the metal ion to which the ligands are attached at the center of a coordination complex. It has a metal ion at its center with a number of other molecules or ions contiguous it. The central ion is the synchronization center, while the molecules or ions bound to it are termed complexing agents or ligands. These can be measured to be attached to the central ion by coordinate bonds. The molecules or ions contiguous the central metal ion are called ligands. Hence the ligands are Lewis based and they surround at least one pair electrons to donate to metal atom/ion with no risk of it. Simple ligands include water, ammonia and chloride ions.
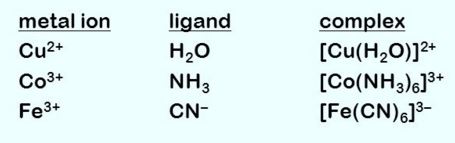
A coordination complex is the products of lewis acids which is based reaction in which nonaligned moles or anion and bond to a central metal atom and also coordinate covalent bonds. Metal complexes can be neutral, positively charged, or negatively charged. Electrically charged metal complexes are sometimes called complexions. A coordination compound contains one or more metal complexes.