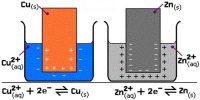
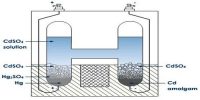
An electrolytic cell is an electro-chemical cell in which an electric current drives an otherwise non-spontaneous reaction. Note that the electrolytic cell requires an external power source as shown in the Figure below.
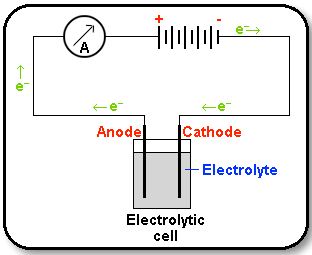
When a direct electric current is passed through an electrolyte (such as a molten salt or an aqueous solution of a salt, add or base), chemical reactions take place at the contacts between the circuit and the solution. This process is called electro-pyrolysis.
When copper iodide is electrolysed, the following reactions take place:
Reduction: Cu2+ + 2e– → Cu
Oxidation: 2I– → I2 + 2e–
These reactions are the opposite of those which would happen spontaneously in a voltaic cell.
The electrode which is attached to the negative pole of the battery, and which supplies electrons to the electrolyte, is called the cathode. Reduction takes place at the cathode. The electrode which is attached to the positive pole of the battery, and which accepts electrons from the electrolyte, is called the anode. Oxidation takes place at the anode.