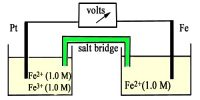
Temperature has a significant effect on most reactions. The position of the equilibrium will shift with a change of temperature. Reaction rates generally increase with an increase in temperature. Consequently, equilibrium is established sooner. Also, the value of the equilibrium constant Kc varies with temperature.
The effect on the position of equilibrium of increasing the temperature (in other words, adding heat) can be predicted using Le Chatelier’s principle. Heat can be treated as if it were another substance being produced or used up in the reaction.
Heat can be treated as if it were a product in exothermic reactions as follows:
Reactants ↔ Products + Heat, ΔH < 0
Increasing temperature is similar to adding more of the product. This will cause the equilibrium to shift in the direction which counteracts the imposed temperature increase by using up heat, i.e. to the left, and therefore increasing the amount of reactants.
Heat behaves as a reactant in endothermic reactions as follows:
Reactants + Heat ↔ Products, ΔH > 0
Increasing temperature is similar to adding more reactant. This will cause the equilibrium to shift to the right, counteracting the imposed temperature change by using up heat, and therefore increasing the amount of products.