Oxidation
According to the classical concept of oxidation, it may be defined as “Oxidation” is a process which involves the addition of oxygen or any other electronegative element or radical to an element or a compound or the removal of hydrogen any electro-positive element or radical from a compound.
For example:
Addition of oxygen or any other electro-negative element
- C + O2 –> CO2
Here, carbon adds oxygen and is thus oxidized to CO2.
- Cu + Cl2 –> CaCl2
Here, chlorine has been added to copper and thus it is oxidized to CuC12.
- 2FeCl2 + Cl2 –> 2FeCl3
In this reaction FeCl2 gains electro-negative atom that is chlorine (Cl–) and is therefore oxidized to FeCl3.
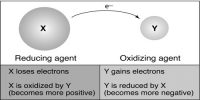
According to the electronic concept oxidation is a process which involves loss of electrons by an atom or group of atoms. Due to this loss of electrons there will be an increase in positive charge and simultaneously will decrease in negative charge.
Example:
Sodium reacts with chlorine forming NaCI.
2Na + Cl2 –> 2NaCI
In the above reaction sodium (Na) looses one electron and thus has become oxidized.
Na –> Na+ + e (Oxidation)