As climate change continues to wreak havoc on our planet, scientists are working to develop more efficient and environmentally friendly energy sources. Biological systems could provide an appealing alternative to common petrochemical processes that emit significant amounts of greenhouse gases and other waste. Recent research has resulted in a greater understanding of biochemical pathways and higher rates of chemical production by biological systems.
Recent work by Michael Jewett of Northwestern Engineering and researchers from the University of Texas at Austin has resulted in a greater understanding of biochemical pathways and increased rates of chemical production by biological systems. The findings may bring us closer to implementing environmentally friendly alternatives to the synthesis of materials, fuels, and other oil-derived products.
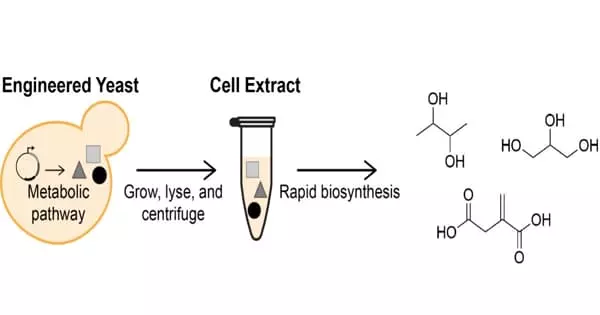
The paper “An Integrated In Vivo/In Vitro Framework to Enhance Cell-Free Biosynthesis with Metabolically Rewired Yeast Extracts,” published in the journal Nature Communications, describes the development of optimized in vitro biosynthesis (biochemical production) processes that use cell extracts from engineered strains of Saccharomyces cerevisiae (brewer’s yeast). Blake Rasor, a Ph.D. student in Jewett’s lab, co-wrote the paper with Jewett, Walter P. Murphy Professor of Chemical and Biological Engineering at the McCormick School of Engineering. The investigation was carried out in collaboration with Hal Alper’s research group at the University of Texas at Austin.
Our work contributes to a growing body of research that seeks to use cell-free systems derived from crude cell extracts for designing a cellular function, on-demand bio-manufacturing, and portable diagnostics.
Blake Rasor
The research was funded by the US Department of Energy Joint Genome Institute’s Emerging Technologies Opportunity Program (ETOP). The ETOP provides funding to kickstart the development of new technologies made available to researchers around the world who use JGI’s user programs to advance energy and environmental applications.
Decades of metabolic research and the development of genetic tools have resulted in S. cerevisiae being a highly controllable framework for biochemical production. This yeast has been engineered to produce a plethora of target molecules used in industrial and therapeutic applications, in addition to its historical applications in baking and brewing.
Cellular production systems, on the other hand, are caught in a tug of war between producing more cells and producing the engineered product. Jewett’s group circumvents these growth and viability constraints by extracting the biological machinery from cells and using the extracted material in cell-free biochemical reactions, allowing them to optimize levers that are difficult to tune in living cells.
Previously, cell-free biosynthesis efforts with crude cell extracts relied primarily on unmodified E. coli strains. The researchers broadened the scope of this technique by incorporating S. cerevisiae extracts and cellular metabolic engineering techniques to improve the biosynthetic potential of cell-free reactions. This demonstrates that metabolic rewiring in cells results in extracts with higher volumetric outputs than wildtype (unaltered) extracts and cell cultures.
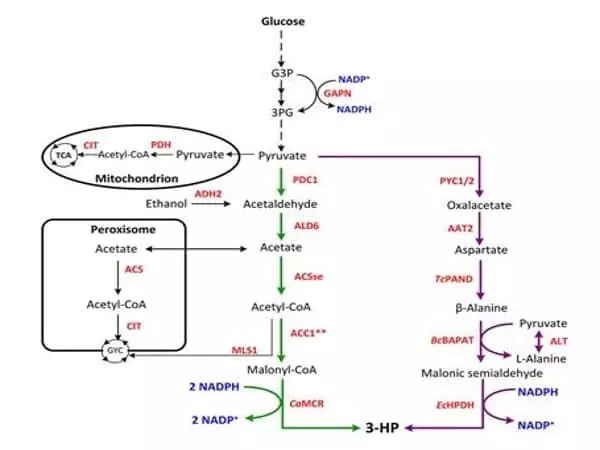
The cell-free production of three chemical products (butanediol, glycerol, and itaconic acid) at up to ten times the rate of corresponding cellular approaches demonstrates the flexibility and efficacy of integrating cellular engineering with cell-free biosynthesis.
“This could broaden the breadth of biological platforms underpinning sustainability efforts,” Rasor said. “Our work contributes to a growing body of research that seeks to use cell-free systems derived from crude cell extracts for designing a cellular function, on-demand bio-manufacturing, and portable diagnostics,” said Jewett, director of the Center for Synthetic Biology. “These efforts, in fact, are broadening the definition of bio-manufacturing in order to build a sustainable bioeconomy.”
In terms of the next steps, Jewett and his colleagues are expanding on this work for pathway prototyping in the context of altered metabolism as well as cell-free bio-manufacturing to supplement current cell-based approaches.
“Extending the integrated cell/cell-free metabolic engineering strategy to yeast strains producing other value-added biochemical products and scaling up cell-free reactions could pave the way for the development of sustainable, economically viable alternatives to current chemical production processes,” he said.
The current global demand for bioethanol is increasing as a result of limited fossil fuel availability and an increase in the number of ethanol/gasoline flex-fuel vehicles. Furthermore, countries around the world have agreed to reduce carbon dioxide emissions, and the use of ethanol as a fuel (which emits fewer pollutants than petroleum products) has been regarded as a viable alternative to petroleum products.