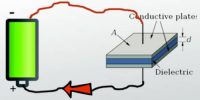
A Leclanche cell consists of a carbon electrode packed in a porous pot containing manganese dioxide and charcoal powder (Figure). The porous pot is immersed in a saturated solution of ammonium chloride (electrolyte) contained in an outer glass vessel. A zinc rod is immersed in electrolytic solution.
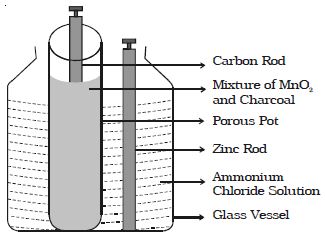
At the zinc rod, due to oxidation reaction Zn atom is converted into Zn++ ions and 2 electrons. Zn++ ions reacting with ammonium chloride produces zinc chloride and ammonia gas.
Zn++ + 2 NH4Cl → 2NH3 + ZnCl2 + 2 H+ + 2e–
The ammonia gas escapes. The hydrogen ions diffuse through the pores of the porous pot and react with manganese dioxide. In this process the positive charge of hydrogen ion is transferred to carbon rod. When zinc rod and carbon rod are connected externally, the two electrons from the zinc rod move towards carbon and neutralizes the positive charge. Thus current flows from carbon to zinc.
Leclanche cell is useful for supplying intermittent current. The emf of the cell is about 1.5 V, and it can supply a current of 0.25 A.